Abstract
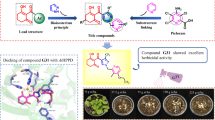
Through tuning and optimizing the phenyl substituents and alkyl length, a series of pyridinium-functionalized amphiphiles possessing potent antibacterial activity toward three types of plant pathogenic bacteria were obtained. Investigations on the inhibition effect of substituents on the phenyl ring towards the bioactivity suggested that the substitutional group was not the crucial factor for the bioactivity. In comparison, the antibacterial effects could be significantly enhanced with increasing the length of alkyl chains. Among these amphiphiles, 6c, 6f, 6h, 6i, 6k, 6l, 6n, and 6q exhibited remarkable inhibition activities against the three pathogenic bacteria with the half-maximal effective concentration (EC50) values within 0.128–1.98 µg/mL. Furthermore, the minimum EC50 values against the pathogens Xanthomonas oryzae pv. oryzae and Xanthomonas axonopodis pv. citri could reach to 0.128 and 0.403 µg/mL, respectively, which were decreased about four times than those of our previous results. Given their simple synthesis and biocidal antibacterial activity, this kind of amphiphiles could be developed as promising bactericides against plant bacterial diseases.


Similar content being viewed by others
References
Bharate SB, Thompson CM (2010) Antimicrobial, antimalarial, and antileishmanial activities of mono- and bis-quaternary pyridinium compounds. Chem Biol Drug Des 76:546–551. doi:10.1111/j.1747-0285.2010.01035.x
Bodor N, Kaminski JJ, Selk S (1980) Soft drugs 1. Labile quaternary ammonium salts as soft antimicrobials. J Med Chem 23:469–474
Brahmachari S, Debnath S, Dutta S, Das PK (2010) Pyridinium based amphiphilic hydrogelators as potential antibacterial agents. Beilstein J Org Chem 6:859–868. doi:10.3762/bjoc.6.101
Chanawanno K, Chantrapromma S, Anantapong T, Kanjana-Opas A, Fun HK (2010) Synthesis, structure and in vitro antibacterial activities of new hybrid disinfectants quaternary ammonium compounds: pyridinium and quinolinium stilbene benzenesulfonates. Eur J Med Chem 45:4199–4208. doi:10.1016/j.ejmech.2010.06.014
Chen Y, Yang X, Gu CY, Zhang AF, Zhang Y, Wang WX, Gao TC, Yao J, Yuan SK (2015) Activity of a novel bactericide, zinc thiazole against Xanthomonas oryzae pv. oryzae in Anhui Province of China. Ann Appl Biol 166:129–135. doi:10.1111/aab.12170
Debnath S, Shome A, Das D, Das PK (2010) Hydrogelation through self-assembly of Fmoc-peptide functionalized cationic amphiphiles: potent antibacterial agent. J Phys Chem B 114:4407–4415. doi:10.1021/jp909520w
Desai NC, Dodiya AM (2014) Synthesis, characterization and in vitro antimicrobial screening of quinoline nucleus containing 1,3,4-oxadiazole and 2-azetidinone derivatives. J Saudi Chem Soc 18:425–431. doi:10.1016/j.jscs.2011.09.005
Desai NC, Kotadiya GM (2014) Microwave-assisted synthesis of benzimidazole bearing 1,3,4-oxadiazole derivatives: screening for their in vitro antimicrobial activity. Med Chem Res 23:4021–4033. doi:10.1007/s00044-014-0978-0
Desai NC, Dodiya AM, Rajpara KM, Rupala YM (2014) Synthesis and antimicrobial screening of 1,3,4-oxadiazole and clubbed thiophene derivatives. J Saudi Chem Soc 18:255–261. doi:10.1016/j.jscs.2011.06.020
Ding D, Boudreau MA, Leemans E, Spink E, Yamaguchi T, Testero SA, O’Daniel PI, Lastochkin E, Chang M, Mobashery S (2015) Exploration of the structure–activity relationship of 1,2,4-oxadiazole antibiotics. Bioorg Med Chem Lett 25:4854–4857. doi:10.1016/j.bmcl.2015.06.044
Fuhrhop JH, Wang TY (2004) Bolaamphiphiles. Chem Rev 104:2901–2938. doi:10.1021/cr030602b
Garudachari B, Isloor AM, Satyanaraya MN, Ananda K, Fun HK (2014) Synthesis, characterization and antimicrobial studies of some new trifluoromethyl quinoline-3-carbohydrazide and 1,3,4-oxadiazoles. RSC Adv 4:30864–30875. doi:10.1039/c4ra04456h
Haldar J, Aswal VK, Goyal PS, Bhattacharya S (2001) Molecular modulation of surfactant aggregation in water: effect of the incorporation of multiple headgroups on micellar properties. Angew Chem Int Ed 40:1228–1232. doi:10.1002/1521-3773(20010401)40:7<1228:AID-ANIE1228>3.3.CO;2-9
Haldar J, Kondaiah P, Bhattacharya S (2005) Synthesis and antibacterial properties of novel hydrolyzable cationic amphiphiles. Incorporation of multiple head groups leads to impressive antibacterial activity. J Med Chem 48:3823–3831. doi:10.1021/jm049106l
Hand LH, Moreland HJ (2014) Surface water mineralization of isopyrazam according to OECD 309: observations on implementation of the new data requirement within agrochemical regulation. Environ Toxicol Chem 33:516–524
Hazra A, Mondal S, Maity A, Naskar S, Saha P, Paira R, Sahu KB, Paira P, Ghosh S, Sinha C, Samanta A, Banerjee S, Mondal NB (2011) Amberlite-IRA-402 (OH) ion exchange resin mediated synthesis of indolizines, pyrrolo [1,2-a] quinolines and isoquinolines: antibacterial and antifungal evaluation of the products. Eur J Med Chem 46:2132–2140. doi:10.1016/j.ejmech.2011.02.066
He K, Yang SY, Li H, Wang H, Li ZL (2014) Effects of calcium carbonate on the survival of Ralstonia solanacearum in soil and control of tobacco bacterial wilt. Eur J Plant Pathol 140:665–675. doi:10.1007/s10658-014-0496-4
Hu J, Wang PY, Lin Y, Zhang J, Smith M, Pellechia PJ, Yang S, Song BA, Wang Q (2014) Self-assembly of pyridinium-functionalized anthracenes: molecular-skeleton-directed formation of microsheets and microtubes. Chem A Eur J 20:7603–7607
Jayatissa RN, Perera RP, Hettiarachchi CM, Weerawarna PM (2012) In vitro antibacterial activity of 4-phenyl-1-(2-phenylallyl)pyridinium bromide: a novel class of pyridinium based antibacterial compounds. Indian J Microbiol 52:83–87. doi:10.1007/s12088-011-0234-y
Kahriman N, Yayli B, Aktas A, Iskefiyeli Z, Beris FS, Yayli N (2013) Synthesis, antibacterial and antioxidant activities of new 1-alkyl-4-(1-alkyl-4-oxo-1,4-dihydroquinolin-2-yl)pyridinium bromides. Eur J Med Chem 69:348–355. doi:10.1016/j.ejmech.2013.08.050
Leemans E, Mahasenan KV, Kumarasiri M, Spink E, Ding DR, O’Daniel PI, Boudreau MA, Lastochkin E, Testero SA, Yamaguchi T, Lee M, Hesek D, Fisher JF, Chang M, Mobashery S (2016) Three-dimensional QSAR analysis and design of new 1,2,4-oxadiazole antibacterials. Bioorg Med Chem Lett 26:1011–1015. doi:10.1016/j.bmcl.2015.12.041
Li P, Yin J, Xu WM, Wu J, He M, Hu DY, Yang S, Song BA (2013) Synthesis, antibacterial activities, and 3D-QSAR of sulfone derivatives containing 1,3,4-oxadiazole moiety. Chem Biol Drug Des 82:546–556. doi:10.1111/cbdd.12181
Li P, Shi L, Gao M, Yang X, Xue W, Jin LH, Hu DY, Song BA (2015) Antibacterial activities against rice bacterial leaf blight and tomato bacterial wilt of 2-mercapto-5-substituted-1,3,4-oxadiazole/thiadiazole derivatives. Bioorg Med Chem Lett 25:481–484. doi:10.1016/j.bmcl.2014.12.038
Muller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, Imazato S, Ruhl S, Schmalz G (2009) Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials 30:4921–4929. doi:10.1016/j.biomaterials.2009.05.079
O’Daniel PI, Peng ZH, Pi HL, Testero SA, Ding DR, Spink E, Leemans E, Boudreau MA, Yamaguchi T, Schroeder VA, Wolter WR, Llarrull LI, Song W, Lastochkin E, Kumarasiri M, Antunes NT, Espahbodi M, Lichtenwalter K, Suckow MA, Vakulenko S, Mobashery S, Chang M (2014) Discovery of a new class of non-beta-lactam inhibitors of penicillin-binding proteins with gram-positive antibacterial activity. J Am Chem Soc 136:3664–3672. doi:10.1021/ja500053x
Parikh K, Joshi D (2014) Synthesis and evaluation of 2-(5-(aryl)-1,3,4-oxadiazol-2-ylthio)-N-(3-(trifluoromethyl)phenyl)acetamides and N-(4-chloro-3-fluorophenyl)-2-(5-(aryl)-1,3,4-oxadiazol-2-ylthio)acetamides as antimicrobial agents. J Chem Sci 126:827–835. doi:10.1007/s12039-014-0625-9
Paw D, Thomas R, Laura K, Karina N, Thomas AM (1994) Estimation of bacterial growth rates from turbidimetric and viable count data. Int J Food Microbiol 23:391–404
Pernak J, Rogoza J, Mirska I (2001) Synthesis and antimicrobial activities of new pyridinium and benzimidazolium chlorides. Eur J Med Chem 36:313–320. doi:10.1016/S0223-5234(01)01226-0
Piazza A, Zimaro T, Garavaglia BS, Ficarra FA, Thomas L, Marondedze C, Feil R, Lunn JE, Gehring C, Ottado J, Gottig N (2015) The dual nature of trehalose in citrus canker disease: a virulence factor for Xanthomonas citri subsp. citri and a trigger for plant defence responses. J Exp Bot 66:2795–2811. doi:10.1093/jxb/erv095
Sambhy V, Peterson BR, Sen A (2008) Antibacterial and hemolytic activities of pyridinium polymers as a function of the spatial relationship between the positive charge and the pendant alkyl tail. Angew Chem Int Ed 47:1250–1254. doi:10.1002/anie.200702287
Shirai A, Ueta S, Maseda H, Kourai H, Omasa T (2012) Action of reactive oxygen species in the antifungal mechanism of gemini-pyridinium salts against yeast. Biocontrol Science 17:77–82
Spink E, Ding DR, Peng ZH, Boudreau MA, Leemans E, Lastochkin E, Song W, Lichtenwalter K, O’Daniel PI, Testero SA, Pi HL, Schroeder VA, Wolter WR, Antunes NT, Suckow MA, Vakulenko S, Chang M, Mobashery S (2015) Structure-activity relationship for the oxadiazole class of antibiotics. J Med Chem 58:1380–1389. doi:10.1021/jm501661f
Stratton TR, Howarter JA, Allison BC, Applegate BM, Youngblood JP (2010) Structure-activity relationships of antibacterial and biocompatible copolymers. Biomacromolecules 11:1286–1290. doi:10.1021/bm1000839
Sundararaman M, Kumar RR, Venkatesan P, Ilangovan A (2013) 1-Alkyl-(N,N-dimethylamino)pyridinium bromides: inhibitory effect on virulence factors of Candida albicans and on the growth of bacterial pathogens. J Med Microbiol 62:241–248. doi:10.1099/jmm.0.050070-0
Wang LY, Qin HL, Wang L, Huo SC, Zhao B, Song B, Yan T (2013) Synthesis and properties of 1-(2-(alkylamino)-2-oxoethyl) pyridinium chloride surfactants. J Chem Res 37:205–207. doi:10.3184/174751913X13626828214397
Wang PY, Hu J, Lin Y, Smith M, Yang S, Song BA, Wang Q (2014a) Microsheets assembled from pyridinium-tailored anthracenes. Tetrahedron 70:6651–6655. doi:10.1016/j.tet.2014.06.084
Wang X, Yin J, Shi L, Zhang G, Song BA (2014b) Design, synthesis, and antibacterial activity of novel Schiff base derivatives of quinazolin-4(3H)-one. Eur J Med Chem 77:65–74. doi:10.1016/j.ejmech.2014.02.053
Wang PY, Zhou L, Zhou J, Wu ZB, Xue W, Song BA, Yang S (2016) Synthesis and antibacterial activity of pyridinium-tailored 2,5-substituted-1,3,4-oxadiazole thioether/sulfoxide/sulfone derivatives. Bioorg Med Chem Lett 26:1214–1217. doi:10.1016/j.bmcl.2016.01.029
Xu WM, Li SZ, He M, Yang S, Li XY, Li P (2013) Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1,2,3-thiadiazole and 1,3,4-oxadiazole/thiadiazole moiety. Bioorg Med Chem Lett 23:5821–5824. doi:10.1016/j.bmcl.2013.08.107
Zeun R, Scalliet G, Oostendorp M (2013) Biological activity of sedaxane—a novel broad-spectrum fungicide for seed treatment. Pest Manag Sci 69:527–534. doi:10.1002/ps.3405
Acknowledgements
We acknowledge the financial support of the Key Technologies R&D Program (2014BAD23B01), National Natural Science Foundation of China (21372052, 21662009), the Research Project of Ministry of Education of China (20135201110005).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
11696_2016_21_MOESM1_ESM.docx
Supplementary data (including experimental section; experimental characterization data of 6a-6q; the related 1H NMR, 13C NMR, and 19F NMR spectra of 6a-6q see Figure S1~S37) associated with this article (xxxxxx) can be found in the online version of this paper (DOI: xxxxxxxxxx). (DOCX 3845 kb)
Rights and permissions
About this article
Cite this article
Wang, PY., Zhou, L., Zhou, J. et al. Potent antibacterial agents: pyridinium-functionalized amphiphiles bearing 1,3,4-oxadiazole scaffolds. Chem. Pap. 71, 1013–1018 (2017). https://doi.org/10.1007/s11696-016-0021-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0021-7