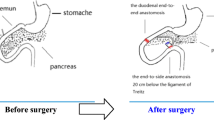
Abstract
Background
The purpose of this study was to investigate the hypoglycemic effect of new biliopancreatic diversion and duodenal–jejunal bypass in Goto–Kakizaki rats and observe effects of the new surgical procedure on the glucose tolerance of GK rats.
Methods
Twenty-four 10-week-old rats (SPF grade) were randomly divided into groups A, B, and C, each with eight rats. Group A underwent duodenal–jejunal bypass, group B underwent modified biliopancreatic diversion, and group C underwent a sham operation. Median rat body weight, fasting blood glucose, OGTT, and blood lipids were measured in fasting 1 week before surgery and 1, 2, 4, and 8 weeks after surgery. Changes in gastric inhibitory polypeptide, glucagon P-like peptide-1, and insulin levels were measured by ELISA 1 week before surgery and 8 weeks after surgery.
Results
Rats’ mean body weight in groups A and B decreased significantly from 368.025 ± 11.726 and 373.100 ± 9.859 g preoperatively to 345.750 ± 11.403 and 343.260 ± 12.399 g at the early postoperative stage (P < 0.05), and with statistically significant differences compared to the weight of rats in group C (P < 0.05). Comparisons between fasting blood glucose before surgery and 8 weeks after surgery revealed no significant differences between all three groups (P > 0.05). Glucose tolerance in groups A and B decreased from preoperative 21.175 ± 3.684 and 20.820 ± 1.671 mmol/L to postoperative 8.950 ± 0.580 and 10.500 ± 1.509 mmol/L, and both were better than that of group C (P < 0.001).
Conclusions
Both new biliopancreatic diversion and duodenal–jejunal bypass improve glucose tolerance of Goto–Kakizaki rats.








Similar content being viewed by others
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–50.
Rubino F, Marescaux J. Effect of duodenal–jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11.
Mason EE. Ilial transposition and enteroglucagon/GLP-1 in obesity (and diabetic?) surgery. Obes Surg. 1999;9:223–8.
Patriti A, Aisa M, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto–Kakizaki rats through an enhanced proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74–85.
Weng S-G. Modified biliopancreatic diversion for GK rats: a proposal for a simpler technique and mechanism research. Obes Surg. 2012;22(6):997–8.
Hanefeld M, Temelkova-Kurktschiev T. The postprandial state and the risk of atherosclerosis. Diabet Med. 1997;14 Suppl 3:S6–S11.
Coutinho M, et al. The relationship between glucose and incident cardiovascular events. Diabetes Care. 1999;22(2):223–40.
O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100(5):899–904.
Speck M, Cho YM, Rubino F, et al. Duodenal–jejunal bypass protects GK rats from beta-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells co-expressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011;300(5):E923–32.
Maedler K, Spinas GA, Dyntar D, et al. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50(1):69–76.
Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276(18):14890–5.
Acknowledgments
We are thankful to the Fujian Medical University for providing place for housed animal. We also thank the First Hospital Affiliated of Fujian Medical University and Fujian Province Institute of Abdominal Surgery for providing place for our research.
Conflict of interest
The authors declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by: the Fujian Provincial Natural Science Foundation, No. 2010J01152
Rights and permissions
About this article
Cite this article
Weng, Sg., Zhang, B., Feng, S. et al. Effects of Modified Biliopancreatic Diversion on Glucose Tolerance of GK Rats. OBES SURG 23, 522–530 (2013). https://doi.org/10.1007/s11695-012-0830-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-012-0830-x