Abstract
Background
Impairment of mitochondrial function plays an important role in obesity and the development of insulin resistance. The aim of this project was to investigate the mitochondrial DNA copy number in human omental adipose tissue with respect to obesity.
Methods
The mitochondrial DNA (mtDNA) content per single adipocyte derived from abdominal omental adipose tissue was determined by quantitative RT-PCR in a group of 75 patients, consisting of obese and morbidly obese subjects, as well as non-obese controls. Additionally, basal metabolic rate and fat oxidation rate were recorded and expressed as total values or per kilogram fat mass.
Results
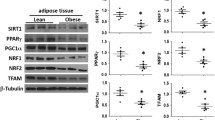
MtDNA content is associated with obesity. Higher body mass index (BMI) resulted in a significantly elevated mtDNA count (ratio = 1.56; p = 0.0331) comparing non-obese (BMI < 30) to obese volunteers (BMI ≥ 30). The mtDNA count per cell was not correlated with age or gender. Diabetic patients showed a trend toward reduced mtDNA content. A seasonal change in mtDNA copy number could not be identified. In addition, a substudy investigating the basal metabolic rate and the fasting fat oxidation did not reveal any associations to the mtDNA count.
Conclusions
The mtDNA content per cell of omental adipose tissue did not correlate with various clinical parameters but tended to be reduced in patients with diabetes, which may partly explain the impairment of mitochondrial function observed in insulin resistance. Furthermore, the mtDNA content was significantly increased in patients suffering from obesity (BMI above 30). This might reflect a compensatory response to the development of obesity, which is associated with impairment of mitochondrial function.
Similar content being viewed by others
References
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359.
Diamond F. The endocrine function of adipose tissue. Growth Genet Horm. 2002;18(2):17–22.
Trayhurn P. Adipocyte biology. Obes Rev. 2007;8(Suppl. 1):41–4.
Maassen JA. Mitochondrial dysfunction in adipocytes: the culprit in type 2 diabetes? Diabetologia. 2006;49(4):619–20.
Choo HJ, Kim JH, Kwon OB, et al. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784–91.
Guilherme A, Virbasius JV, Czech MP, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–77.
Petersen KF, Befroy D, Shulman GI, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;500(5622):1140–2.
Civitarese AE, Ravussin E. Minireview: mitochondrial energetics and insulin resistance. Endocrinology. 2007;149(3):950–4.
Ernster L, Schatz G. Mitochondria: a historical review. J Cell Biol. 1981;91(3):227s–55.
Kuroshima A. Brown adipose tissue thermogenesis as a physiological strategy for adaptation. Jpn J Physiol. 1993;43(43):117.
Justo R, Oliver J, Gianotti M. Brown adipose tissue mitochondrial subpopulations show different morphological and thermogenic characteristics. Mitochondrion. 2005;5(1):45–53.
Collins TJ, Bootman MD. Mitochondria are morphologically heterogenous within cells. J Exp Biol. 2003;206:1993–2000.
Menziens RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1970;246(8):2425–9.
Masuyama M, Iida R, Matsuki T, et al. Quantitative change in mitochondrial DNA content in various mouse tissues during aging. Biochim Biophys Acta. 2005;1723:302–8.
Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–68.
Wu Z, Puigserver P, Spiegelman BM, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–24.
van Marken Lichtenbelt WD, Vanhommering JW, Jaap Teule GJ, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8.
Chan DC. Mitochondrial fusion and fission in mammals. Ann Rev Cell Dev Biol. 2006;22:79–99.
Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–9.
Mingrone G, Manco M, Zorzano A, et al. Could the low level of expression of the gene encoding skeletal muscle mitofusin-2 account for the metabolic inflexibility of obesity? Diabetologia. 2005;48:2108–14.
Bach D, Pich S, Zorzano A, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. J Biol Chem. 2003;278(19):17190–7.
Wilson-Fritch L, Nicoloro S, Corvera S, et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114(9):1281–9.
Peterli R, Wöllnerhanssen B, von Flüe M, et al. Prospective study of a two-stage operative concept in the treatment of morbid obesity: primary Lap-Band followed if needed by sleeve gastroectomy with duodenal switch. Obes Surg. 2007;17:334–40.
Woelnerhanssen B, Kern B, Peterli R, et al. Predictors of outcome in treatment of morbid obesity by laparoscopic adjustable gastric banding: results of a prospective study of 380 patients. Surgery of Obesity and Related Disease. 2008;4:500–6.
Bogacka I, Xie H, Smith SR, et al. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–9.
Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. Springer: New York; 2000.
Barazzoni R, Short KR, Sreekumaran Nair K. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. JBC. 2000;275(5):3343–7.
Colom B, Alcolea MP, Garcia-Palmer FJ, et al. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell Physiol Biochem. 2007;19:205–12.
Mollica MP, Lionetti L, Iossa S, et al. Cold exposure differently influences mitochondrial energy efficiacy in rat liver and skeletal muscle. FEBS Lett. 2005;579:1978–82.
Needergard J, Bengtssen T. Unexpected evidence of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–52.
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738.
Maassen JA. Mitochondrial diabetes: pathophysiology, clinical presentation, and genetic analysis. Am J Med Gen (Semin. Med. Genet.). 2002;115:66–70.
Marcuello A, González-Alonso J, Díez-Sánchez C, et al. Skeletal muscle mitochondrial DNA content in exercising humans. J Appl Physiol. 2005;99:1372–7.
Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95.
Kaaman M, Sparks LM, Arner P, et al. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia. 2007;50:2526–33.
Miller FJ, Rosenfeldt FL, Nagley P, et al. Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR-based assay: lack of changes of copy number with age. Nucleic Acids Res. 2003;31(11):e61.
Rajala MW, Scherer PE. Minireview: the adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144(9):3765–73.
Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nat Med. 2006;444:847–53.
Ferranti SD, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–55.
Robin ED, Wong R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol. 1988;136:507–13.
Cypess AM, Lehmann S, Kahn CR, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17.
Virtanen KA, Lidell ME, Nuutila P, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25.
Befroy DE, Petersen KF, Shulman GI, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–81.
Hellmér J, Marcus C, Arner P, et al. Mechanisms for differences in lipolysis between human subcutaneous and omental fat cells. J Clin Endocrinol Metab. 1992;75(1):15–20.
van Harmelen V, Dicker A, Arner P, et al. Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes. 2002;51:2029–36.
Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–50.
Arvidsson E, Blomqvist L, Ryden M. Depot-specific differences in perilipin mRNA but not protein expression in obesity. J Intern Med. 2004;255:595–601.
Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance. Diabetes. 2000;49:677–83.
Capkova M, Houstek J, Zeman J, et al. Activities of cytochrome c oxidase and citrate synthase in lymphocates of obese and normal-weight subjects. IJO. 2002;26:1110–7.
Kim JY, Hickner RC, Houmard JA, et al. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocriol Metab. 2000;279:1039–44.
Crescenzo R, Bianco F, Iossa S, et al. Alterations in hepatic mitochondrial compartment in a model of obesity and insulin resistance. Obesity. 2008;16:958–64.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors hereby disclose any commercial interest in this present study. We thank the European Society of Pediatric Endocrinology for financial support by their donation of the 2007 IPSEN Collaborative Project Grant.
Rights and permissions
About this article
Cite this article
Lindinger, A., Peterli, R., Peters, T. et al. Mitochondrial DNA Content in Human Omental Adipose Tissue. OBES SURG 20, 84–92 (2010). https://doi.org/10.1007/s11695-009-9987-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-009-9987-3