Abstract
Conceptualisation of evolution requires new inclusions, as evidenced by contributions brought by evolutionary developmental biology—the evo-devo connection. Integration of teratology in an evolutionary framework fits in this continuity. It highlights the production of developmental anomalies (more or less drastic) over evolutionary times, which become integral parts of groups and taxa. Originating in Étienne Geoffroy Saint-Hilaire’s work, the contemporary independent formulation of evolutionary teratology allows a better understanding of some anatomical structures. The limbs of tetrapods are a promising field of study as some changes in their shapes, proportions and compositions are close to malformations observed in teratology. The forelimbs condition of Tyrannosauridae and Carnotaurinae is a good example. They are theropod dinosaurs characterised by different anterior micromelias, codified following an anatomical nomenclature. An association with the knowledge from developmental biology helps to discern possible productive mechanisms of such micromelias, including the influence of developmental rates, Hox genes, growth factors and developmental pathways conserved in evolution. What is more, the case of Tyrannosauridae and Carnotaurinae imposes to use the ‘adaptive’ reasoning in a more balanced framework. Indeed, the viability and evolutionary success of an organism is the result of the equilibrium of aptitudes between its various anatomical parts interacting with the circumstances.









Similar content being viewed by others
Notes
The function of an element or combination of elements (an anatomical structure) represents the achievable mechanical aptitude(s)—movements, rotations, flexions, etc.—authorised by the shape and connections. From this/these biomechanical aptitude(s) derive(s) the possible use(s).
There have been successive changes of name in the literature, according to two variables of genera, namely Tarbosaurus bataar, Tarbosaurus efremovi and Tyrannosaurus bataar. Following results from Currie et al. (2003), Currie (2003b), Hurum and Sabath (2003), the original naming Tarbosaurus has been chosen here.
Validity of Majungasaurus crenatissimus over the name Majungatholus atopus (Krause et al. 2007).
In order to operate significative comparisons, taking into account the fossil preservation factor, it was chosen to represent the autopod only with the median metatacarpal (as Middleton and Gatesy 2000). However, for some potential manual morphologies or conservation issues, it is worth noting that the median metacarpal can be absent or too small compared to other digital rays: in such cases, the dominant developed or avalaible matacarpal (bearing phalanges) will be used.
The term ‘acromesomelic’ describes a group of dysplasia where the limb shortening is more pronounced at the middle and distal segments. An example is the Hunter-Thompson acromesomelic dysplasia (Manouvrier-Hanu et al. 1999).
The manus of Carnotaurus has abnormally short digits, characterising a brachydactyly: “abnormal shortness of one or more fingers or toes” (Dictionnaire de l’Académie de médecine, version 2013, online resource—personal translation from the original French text) or shortening of the fingers due to abnormal development of the phalanges and/or metacarpals (Schwabe et al. 2000). However, the name ‘acromesomelic’ already suggests a more important shortening of the autopod and therefore digits. The choice has been made to highlight only the incomplete development of phalangeal elements—i.e. hypophalangia—to avoid unnecessary and partially redundant term.
References
Abzhanov, A., & Kaufman, T. C. (1999). Novel regulation of the homeotic gene Scr associated with a crustacean leg-to-maxilliped appendage transformation. Development, 126, 1121–1128.
Adamska, M., MacDonald, B. T., Sarmast, Z. H., Oliver, E. R., & Meisler, M. H. (2004). En1 and Wnt7a interact with Dkk1 during limb development in the mouse. Developmental Biology, 272, 134–144.
Agnolin, F. L., & Chiarelli, P. (2010). The position of claws in Naosauridae (Dinosauria: Abelisauroidea) and its implication for abelisauroid manus evolution. Paläontologishe Zeitscrift, 84, 293–300.
Alberch, J. (1989). The logic of monsters: Evidence for internal constraint development and evolution. Geobios, 12(2), 21–57.
Al-Qattan, M. M., Yang, Y., & Kozin, S. H. (2009). Embryology of the upper limb. Journal of Hand Surgery, 34A, 1340–1350.
Alroy, J., & Garcia Selles, A. (2014). Measurements of Deinonychus antirrhopus, Guanlong wucaii, Dilophosaurus weitherilli, Ceratosaurus nasicornis, Ornithomimus edmonticus, Struthiomimus altus, Allosaurus fragilis, Gigantoraptor erlianensis, Herrerasaurus ischigualastensis, Acrocanthosaurus atokensis, Daspletosaurus torosus recorded in the Paleobiology database. Resource document. http://fossilworks.org. Accessed January 2014.
Bakker, R. T., Williams, M., & Currie, P. J. (1988). Nanotyrannus, a new genus of pygmy tyrannosaur, from the latest Cretaceousof Montana. Hunteria, 1, 1–30.
Bates, K. T., & Falkingham, P. L. (2012). Estimating maximum bite performance in Tyrannosaurus rex using multi-body dynamics. Biology Letters, 8, 660–664.
Bever, G. S., Gauthier, J. A., & Wagner, G. P. (2011). Finding the frame shift: Digit loss, developmental variability, and the origin of the avian hand. Evolution and Development, 13(3), 269–279.
Bininda-Emonds, O. R. P., Jeffery, J. E., Sánchez-Villagra, M. R., Hanken, J., Colbert, M., Pieau, C., et al. (2007). Forelimb–hindlimb developmental timing changes across tetrapod phylogeny. BMC Evolutionary Biology, 7, 182.
Bonaparte, J. F., Novas, F. E., & Coria, R. A. (1990). Carnotaurus sastrei Bonaparte, the horned, lightly built carnosaur from the middle cretaceous of Patagonia. In Contributions in science, Natural History Museum of Los Angeles County, vol. 416, pp. 1–42.
Boulet, A. M., & Capecchi, M. R. (2004). Multiple roles of Hoxa11 and Hoxd11 in the formation of the mammalian forelimb zeugopod. Development, 131, 299–309.
Brochu, C. A. (2000). A digitally-rendered endocast for Tyrannosaurus rex. Journal of Vertebrate Paleontology, 20(1), 1–6.
Brochu, C.A. (2003). Osteology of Tyrannosaurus rex: Insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull. Journal of Vertebrate Paleontology 22, Supplement to number 4.
Brusatte, S. L., Carr, T. D., Erickson, G. M., Bever, G. S., & Norell, M. A. (2009). A long-snouted, multihorned tyrannosaurid from the Late Cretaceous of Mongolia. Proceedings of the National Academy of Sciences, 106(41), 17261–17266.
Brusatte, S. L., Norell, M. A., Carr, T. D., Erickson, G. M., Hutchinson, J. R., Balanoff, A. M., et al. (2010). Tyrannosaur paleobiology: New research on ancient exemplar organisms. Science, 139, 1481–1485.
Brusatte, S. L., Benson, R. B. J., & Norell, M. A. (2011). The anatomy of Dryptosaurus aquilunguis (Dinosauria: Theropoda) and a review of its Tyrannosauroid affinities. American Museum Novitates, 3717, 1–53.
Brusatte, S. L, Carr, T. D., & Norell, M. A. (2012). The osteology of Alioramus, a gracile and long-snouted Tyrannosaurid (Dinosauria: Theropoda) from the Late Cretaceous of Mongolia. Bulletin of the American Museum of Natural History, 366, 1–197.
Buckland, R. A., Collinson, J. M., Graham, E., Davidson, D. R., & Hill, R. E. (1998). Antagonistic effects of FGF4 on BMP induction of apoptosis and chondrogenesis in the chick limb bud. Mechanisms of Development, 71, 143–150.
Burch, S. H., & Carrano, M. T. (2012). An articulated pectoral girdle and forelimb of the Abelisaurid theropod Majungasaurus crenatissimus from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology, 32(1), 1–16.
Burtch, R. L. (1966). Nomencalture for congenital skeletal limb deficiencies, a revision of the Frantz and O’Rahilly classification. Artificial Limbs, 10(1), 24–35.
Carabajal, A. P. (2011a). The braincase anatomy of Carnotaurus sastrei (Theropoda: Abelisauridae) from the Upper Cretaceous of Patagonia. Journal of Vertebrate Paleontology, 31(2), 378–386.
Carabajal, A. P. (2011b). Braincases of abelisaurid theropods from the Upper Cretaceous of north Patagonia. Palaeontology, 54(4), 793–806.
Carbone, C., Turvey, S. T., & Biebly, J. (2011). Intra-guild competition and its implications for one of the biggest terrestrial predators, Tyrannosaurus rex. Proceedings of the Royal Society B, 278, 2682–2690.
Carpenter, K., & Smith, M. (2001). Forelimb osteology and biomechanics of Tyrannosaurus rex. In D. Tanke & K. Carpenter (Eds.), Mesozoic vertebrate life (pp. 90–116). Bloomington & Indianapolis: Indiana University Press.
Carr, T. D. (1999). Carnofacial ontogeny in Tyrannosauridae (Dinosaurisa, Coelurosauria). Journal of Vertebrate Paleontology, 19(3), 497–520.
Carr, T. D., & Williamson, T. E. (2010). Bistahieversor sealeyi, gen. et sp. nov., a new Tyrannosauroid from New Mexico and the origin of deep snouts in Tyrannosauroidea. Journal of Vertebrate Paleontology, 30(1), 1–16.
Carr, T. D., Williamson, T. E., Britt, B., & Stadtman, K. (2011). Evidence for high taxonomic and morphologic tyrannosauroid diversity in the Late Cretaceous (Late Campanian) of the American Southwest and a new short-skulled tyrannosaurid from the Kaiparowits formation of Utah. Naturwissenschaften, 98(3), 241–246.
Carrano, M. T. (2007). The appendicular skeleton of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology, 27(2), 163–179.
Carrano, M. T., & Hutchinson, J. R. (2002). Pelvic and hindlimb musculature of Tyannosaurus rex (Dinosauria: Theropoda). Journal of Morphology, 253, 207–228.
Chouard, T. (2010). Evolution: Revenge of the hopeful monster. Nature, 463, 864–867.
Chung, M. S. (2011). Congenital differences of the upper extremity: Classification and treatment principles. Clinics in Orthopedic Surgery, 3, 172–177.
Clark, J. (2009). Becoming T. rex. Science, 326, 373–374.
Coria, R. A., Chiappe, L. M., & Dingus, L. (2002). A new close relative of Carnotaurus sastrei Bonaparte 1985 (Theropoda: Abelisauridae) from the late cretaceous of Patagonia. Journal of Vertebrate Paleontology, 22(2), 460–465.
Crossley, P. H., Minowada, G., MacArthur, C. A., & Martin, G. R. (1996). Roles for FGF8 in the induction, initiation and maintenance of chick limb development. Cell, 84, 127–136.
Currie, P. J. (2003a). Cranial anatomy of tyrannosaurid dinosaurs from the Late Cretaceous of Alberta, Canada. Acta Palaeontologica Polonica, 48(2), 191–226.
Currie, P. J. (2003b). Allometric growth in tyrannosauids (Dinosauria: Theropoda) from the Upper Cretaceous of North America and Asia. Canadian Journal of Earth Sciences, 40, 651–665.
Currie, P. J., & Carpenter, K. (2000). A new specimen of Acrocanthosaurus atokensis (Theropoda, Dinosauria) from the Lower Cretaceous Antlers formation (Lower Cretaceous, Aptian) of Oklahoma, USA. Geodiversitas, 22(2), 207–246.
Currie, P. J., Hurum, J. H., & Sabath, K. (2003). Skull structure and evolution in tyrannosaurid dinosaurs. Acta Palaeontologica Polonica, 48(2), 227–234.
Davis, A. P., & Capecchi, M. R. (1996). A mutational analysis ofthe 5′HoxD genes: Dissection of genetic interactions during limb development in the mouse. Development, 122, 1175–1185.
Day, H. J. B. (1991). The ISO/ISPO classification of congenital limb deficiency. Prosthetics and Orthotics International, 15, 67–69.
de Bakker, M. A. G., Fowler, D. D., den Oude, K., Dondrop, E. M., Garrido Navas, M. C., Horbanczuk, J. O., et al. (2013). Digit loss in archosaur evolution and the interplay between selection and constraints. Nature, 500, 445–449.
Dececchi, T. A., & Larsson, C. E. (2013). Body and limb size dissociation at the origin of birds: Uncoupling allometric constraints across a macroevolutionary transition. Evolution, 67(9), 2741–2752.
DePalma, R. A, I. I., Burnham, D. A., Martin, L. D., Rotschild, B. M., & Larson, P. L. (2013). Physical evidence of predatory behavior in Tyrannosaurus rex. Proceedings of the National Academy of Sciences of the United States of America, 110(31), 12560–12564.
Dictionnaire de l’Académie de medicine. (2013). Resource document. http://dictionnaire.academie-medecine.fr/. Accessed July 2013.
Dietrich, M. R. (2003). Richard Goldschmidt: Hopeful monsters and other ‘heresies’. Nature Reviews Genetics, 4(1), 68–74.
Dittrich-Reed, D. R., & Fitzpatrick, B. (2013). Transgressive hybrids as hopeful monsters. Evolutionary Biology, 40, 310–315.
Entin, M. A. (1959). Reconstruction of congenital abnormalities of the upper extremities. Journal of Bone and Joint Surgery, 41A(4), 681–701.
Ezcurra, M. D., Agnolin, F. L., & Novas, F. E. (2010). An abeliusauroid dinosaur with non-atrophied manus from the Late Cretaceous Pari Aike formation of southern Patagonia. Zootaxa, 2450, 1–25.
Favier, B., & Dollé, P. (1997). Developmental functions of mammalian Hox genes. Molecular Human Reproduction, 3(2), 115–131.
Favier, B., Rijli, F. M., Fromental-Ramain, C., Fraulob, V., Chambon, P., & Dollé, P. (1996). Functional cooperation between the non-paralogous genes Hoxa-10 and Hoxd-11 in the developing forelimb and axial skeleton. Development, 122, 449–460.
Fiorillo, A. R., & Tykoski, R. S. (2014). A diminutive new Tyrannosaur from the tope of the world. PLoS One, 9(3), e91287.
Fowler, D. W., Woodward, H. N., Freedman, E. A., Larson, P. L., & Horner, J. R. (2011). Reanalysis of “Raptorex kriegsteini”: A juvenile Tyrannosaurid dinosaur from Mongolia. PLoS One, 6(6), e21376.
Francis-West, P. H., Abdelfattah, A., Chen, P., Allen, C., Parish, J., Ladher, R., et al. (1999). Mechanisms of GDF-5 action during skeletal development. Development, 126, 1305–1315.
Frantz, C. H., & O’Rahilly, R. (1961). Congenital skeletal limb deficiencies. The Journal of Bone and Joint Surgery, 43A(8), 1202–1224.
Geoffroy Saint-Hilaire, É. (1822). Philosophie anatomique—Des monstruosités humaines, Ouvrage contenant une classification des monstres; une histoire raisonnée des phénomènes de la monstruosité et des faits primitifs qui la produisent; des vues nouvelles touchant la nutrition du fœtus et d’autres circonstances de son développement; et la détermination des diverses parties de l’organe sexuel, pour en démontrer l’unité de composition, non seulement chez les monstres, où l’altération des formes rend cet organe méconnaissable, mais dans les deux sexes, et, de plus, chez les oiseaux et chez les mammifères. Paris: l’auteur.
Geoffroy Saint-Hilaire, É. (1825). Recherches sur l’organisation des gavials; Sur leurs affinités naturelles, desquelles résulte la nécessité d’une autre distribution générique, Gavialis, Teleosaurus et Steneosaurus; et sur cette question, si les Gavial (Gavialis), aujourd’hui répandus dans les parties orientales de l’Asie, descendent, par voie non interrompue de génération, des Gavials antidiluviens [sic], soit des Gavials fossiles, dits Crocodiles de Caen (Teleosaurus), soit des Gavials fossiles du Havre et de Honfleur (Steneosaurus). In Mémoires du Muséum d’Histoire Naturelle, par les professeurs de cet établissement, Tome 12 (pp. 97–155). Paris: Belin.
Geoffroy Saint-Hilaire, É. (1826). Considérations générales sur les monstres comprenant une théorie des phénomènes de la monstruosité. Paris: Tatsu.
Geoffroy Saint-Hilaire, I. (1832). Histoire générale et particulière des anomalies de l’organisation chez l’homme et les animaux, ouvrage comprenant des recherches sur les caractères, la classification, l’influence physiologique et pathologique, les rapports généraux, les lois et les causes des monstruosités, des variétés et des vices de conformation, ou traité de tératologie. Tome premier. Paris: Baillière.
Geoffroy Saint-Hilaire, I. (1841). Essais de zoologie générale, ou mémoires et notices sur la zoologie générale, l’anthropologie, et l’histoire de la science. Paris: Roret.
Giffin, E. B. (1995). Postcranial paleoneurology of the Diapsida. Journal of Zoology, 235, 389–410.
Gilmore, C. W. (1920). Osteology of the carnivorous dinosauria in the United States National Museum, with special reference to the genera Antrodemus (Allosaurus) and Ceratosaurus. Bulletin of the National Museum 110. Washington, Government printing office.
Glut, D. F. (2008). Tyrannosaurus rex: A century of celebrity. In P. L. Larson & K. Carpenter (Eds.), Tyrannosaurus rex, the tyrant king (pp. 398–427). Bloomington and Indianapolis: Indiana University Press.
Gold, N. B., Westgate, M. N., & Holmes, L. B. (2011). Anatomic and etiological classification of congenital limb deficiencies. American Journal of Medical Genetics A, 155A(6), 1225–1235.
Goldshmidt, R. (1940). The material basis of evolution. New Haven: Yale University Press.
Gould, S. J. (1974). The origin of function of “bizarre” structures: Anter size and skull size of the “Irish elk”, Megaloceros giganteus. Evolution, 28(2), 191–220.
Gould, S. J. (1977). The return of hopeful monsters. Natural History, 86(6), 22–30.
Gould, S. J., & Lewontin, R. C. (1979). The Spandrels of San Marco and the Panglossian Paradigm: A critique of the adpatationist programme. Proceedings of the Royal Society of London. Series B: Biological Sciences, 205(1161), 581–598.
Guinard, G. (2012). Evolutionary concepts meet the neck of penguins (Aves: Sphenisciformes), towards a “survival strategy” for evo-devo. Theory in Biosciences, 131(4), 231–242.
Guinard, G., & Marchand, D. (2010). Modularity and complete natural homeoses in cervical vertebrae of extant and extinct penguins (Aves: Sphenisciformes). Evolutionary Biology, 37(4), 210–226.
Guinard, G., Marchand, D., Courant, F., Gauthier-Clerc, M., & Le Bohec, C. (2010). Morphology, ontogenesis and mechanics of cervical vertebrae in four species of penguins (Aves: Spheniscidae). Polar Biology, 33(6), 807–822.
Hall, B. K. (2002). Palaeontology and evolutionary developmental biology: A science of the nineteenth and twenty-first centuries. Palaeontology, 45(4), 647–669.
Heslop-Harrison, J. (1952). A reconsideration of plant teratology. Phyton, 4, 19–34.
Holtz, T. R. (2001). The phylogeny and taxonomy of the Tyrannosauridae. In D. H. Tanke & K. Carpenter (Eds.), Mesozoic vertebrate life (pp. 64–83). Bloomington and Indianapolis: Indiana University Press.
Hone, D. E., Wang, K., Sullivan, C., Zhao, X., Chen, S., Li, D., et al. (2011). A new, large tyrannosaurine theropod from the Upper Cretaceous of China. Cretaceous Research, 32(4), 495–503.
Huang, C., & Hales, B. F. (2009). Teratogen responsive signaling pathways in organogenesis stage mouse limbs. Reproductive Teratology, 27, 103–110.
Huang, R., Zhi, Q., Patel, K., Wilting, J., & Christ, B. (2000). Dual origin and segmental organisation of the: Avian scapula. Development, 127, 3789–3794.
Hurum, J. H., & Currie, P. J. (2000). The crushing bites of Tyrannosaurids. Journal of Vertebrate Paleontology, 20(3), 619–621.
Hurum, J. H., & Sabath, K. (2003). Giant theropod dinosaurs from Asia and North America: Skulls of Tarbosaurus bataar and Tyrannosaurus rex compared. Acta Palontologica Polonica, 48(2), 161–190.
Hutchinson, J. R., Bates, K. T., Molnar, J., Allen, V., & Makovicky, P. J. (2011). A computational analysis of limb and body dimensions in Tyrannosaurus rex with implication for locomotion, ontogeny and growth. PLoS One, 6(10), e26037.
Hutt, S., Naish, D., Martill, D. M., Barker, M. J., & Newbery, P. (2001). A preliminary account of a new tyrannosauroid theropod from the Wessex Formation (Cretaceous) of southern England. Cretaceous Research, 22, 227–242.
Jeanty, P., & Valero, G. (2012). The assessment of the fetus with a skeletal dysplasia. Resource document. http://www.sonoworld.com/Client/Fetus/files/skeletal_eng.pdf. Accessed August 17, 2012.
Jelínek, R. (2005). The contribution of new findings and ideas to the old principles of teratology. Reproductive Toxicology, 20, 295–300.
Johnson, R. L., & Tabin, C. J. (1997). Molecular models for vertebrate limb development. Cell, 90, 979–990.
Knell, R. J., & Sampson, S. (2011). Bizarre structures in dinosaurs: Species recognition or sexual selection? A response to Padian and Horner. Journal of Zoology, 283, 18–22.
Kozin, S. H. (2003). Upper-extremity congenital anomalies. Bone and Joint Surgery, 85(8), 1564–1576.
Krause, D. W., Sampson, S. D., Carano, M. T., & O’Connor, P. M. (2007). Overview of the history of discovery, taxonomy, phylogeny, and biogeography of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the late Cretaceous of Madagascar. Journal of Vertebrate Paleontology, 27 (supplement 2), 1–20.
Kutschera, U., & Niklas, K. J. (2008). Macroevolution via secondary endosymbiosis: A Neo-Goldschmidtian view of unicellular hopeful monsters and Darwin’s primordial intermediate form. Theory in Biosciences, 127, 277–289.
Lambe, L. M. (1914a). On the fore-limb of a carnivorous dinosaur from the belly river formation of Alberta, and a new genus of Ceratopsia from the same horizon, with remarks on the integument of some Cretaceous herbivorous dinosaurs. The Ottawa Naturalist, 27, 129–135.
Lambe, L. M. (1914b). On a new genus and species of carnivorous dinosaur from the belly river formation of Alberta, with the description of the skull of Stephanosaurus marginatus from the same horizon. The Ottawa Naturalist, 28, 13–20.
Larson, P. (2013). The validity of Nanotyrannus Lancensis (Theropoda, Lancian—Upper Maastrichtian of North America). In Society of paleontology: 73rd annual meeting, abstracts with programs, vol. 159.
Larsson, H. C. E., & Wagner, G. P. (2003). Old morphologies misinterpreted. Trends in Ecology & Evolution, 18(1), 10.
Lefebvre, B. (2003). Stephen J. Gould, les mitrates et les monstres. Comptes Rendus Palevol, 2, 509–522.
Light, T. R. (1989).Congenital malformations and deformities of the hand—part A: General concepts. In J. S. Barr Jr. (Ed.), Instructional course lectures, American Academy of Orthopedic Surgeons, (Vol. 38, pp. 31–34).
Lipkin, C., & Carpenter, K. (2008). Looking again at the forelimb of Tyrannosaurus rex. In P. Larson & K. Carpenter (Eds.), Tyrannosaurus rex, the tyrant king (pp. 166–189). Bloomington and Indianapolis: Indiana University Press.
Lockley, M., Kuhihara, R., & Mitchell, L. (2008). Why Tyrannosaurus rex had puny arms: An integral morphodynamic solution to a simple puzzle in Theropod Paleobiology. In P. Larson & K. Carpenter (Eds.), Tyrannosaurus rex, the tyrant king (pp. 130–164). Bloomington and Indianapolis: Indiana University Press.
Loewen, M. A., Irmis, R. B., Sertich, J. J. W., Currie, P. J., & Sampson, S. D. (2013). Tyrant dinosaur evolution tracks the rise and fall of Late Cretaceous Oceans. PLoS One, 8(11), e79420.
Lü, J., Yi, L., Brusatte, S. L., Yang, L., Li, H., & Liu, C. (2014). A new clade of Asian Late Cretaceous long-snouted tyrannosaurids. Nature Communications, 5, 3788.
Lyons, K., & Ezaki, M. B. (2009). Molecular regulation of limb growth. Journal of Bone and Joint Surgery (Amercian Volume), 91(Supplement 4), 47–52.
Mahler, L. (2005). Record of Abelisauridae (Dinosauria: Theropoda) from the Cenomanian of Morocco. Journal of Vertebrate Paleontology, 25(1), 236–239.
Maleev, E. A. (1974). Gigantic carnosaurs of the family Tyrannosauridae. The Joint Soviet-Mongolian Paleontological Expedition Transactions, 1, 132–191.
Manouvrier-Hanu, S., Holder-Espinasse, M., & Lyonnet, S. (1999). Genetics of limbs anomalies in humans. Trends if Genetics, 15(10), 409–417.
Marsh, O. C. (1892). Restorations of Claosaurus and Ceratosaurus. American Journal of Science, 44(262), 343–349.
Marsh, O. C. (1896). The Dinosaurs of North America. In C. D. Walcott (Ed.), Sixteenth annual report of the United States Geological Survey to the Secretary of Interior 1894–1895, part 1.—Director’s report and papers of a theoretic nature (pp. 143–468). Washington: Government Printing Office.
Matthew, W. D., & Brown, B. (1923). Preliminary notices of skeletons and skulls of Deinodontidae from the Cretaceous of Alberta. American Museum Novitates, 89, 1–9.
Mazzetta, G. V., Farina, R. A., & Vizcaino, S. F. (1998). On the palaebiology of the South American horned theropod Carnotaurus sastrei Bonaparte. Gaia, 15, 185–192.
Mazzetta, G. V., Christiansen, P., & Farina, R. A. (2004). Giants and bizarre: Body size of some southern South American Cretaceous dinosaurs. Historical Biology, 16(2–4), 71–83.
Meckel, J. F. (1815). De duplicate monstrosa commentarius. Halae & Beroloni: E. Librariis orphanothrophei Berlin: 53–54. Comment no. 49.
Méndez, A. H. (2014a). The caudal vertebral series in abelisaurid dinosaurs. Acta Palaeontologica Polonica, 59(1), 99–107.
Méndez, A. H. (2014b). The cervical vertebrae of the Late Cretaceous abelisaurid dinosaur Carnotaurus sastrei. Acta Palaeontologica Polonica, 59(3), 569–579.
Middleton, K. M., & Gatesy, S. M. (2000). Theropod forelimb design and evolution. Zoological Journal of the Linnean Society, 128, 149–187.
Moeller, C., Swindell, E. C., Kispert, A., & Eichele, G. (2003). Carboxypeptidase Z (CPZ) modulates Wnt signaling and regulates the development of skeletal elements in the chicken. Development, 130, 5103–5111.
Morgan, B. A. (1997). Hox genes and embryonic development. Poultry Science, 76, 96–104.
Nelson, C. E., Morgan, B. A., Burke, A. C., Laufer, E., DiManbro, E., Murtaugh, C. L., et al. (1996). Analysis of Hox gene expression in the chick limb bud. Development, 122, 1449–1466.
Niederreither, K., Vermot, J., Schuhbaur, B., Chambon, P., & Dollé, P. (2002). Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development, 129, 3563–3574.
Novas, F. E., Ezcurra, M. D., & Agnolin, F. (2006). Humerus of a basal abelisauroid theropod from the Late Cretaceous of Patagonia. Revista del Museo Argentino de Ciencias Naturales, 8(1), 63–68.
O’Connor, P. M. (2007). The postcranial axial skeleton of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. Journal of Paleontology, 27(Supplement to No 2), 127–162.
Osmólska, H., & Roniewicz, E. (1970). Deinocheiridae, a new family of theropod dinosaurs. Palaeontologica Polonica, 21, 5–19.
Osmólska, H., Roniewicz, E., & Barsbold, R. (1972). A new dinosaur, Gallimimus bullatus n.gen., n.sp. (Ornithomimidae) from the Upper Cretaceous of Mongolia. Palaeontologica Polonica, 27, 103–143.
Ostrom, J. H. (1969). Osteology of Deinonychus antirrhopus, and unusual Theropod from the Lower Cretaceous of Montana. In Bulletin of the Peabody Museum of Natural History, (Vol. 30, pp. 1–65). New Haven, CT: Yale University.
Padian, K., & Horner, J. R. (2011). The evolution of ‘bizarre structures’ in dinosaurs: Biomechanics, sexual selection, social selection or species recognition? Journal of Zoology, 283, 3–17.
Panman, L., Drenth, T., Tewelscher, P., Zuniga, A., & Zeller, R. (2005). Genetic interaction between Gli3 and Alx4 during limb and craniofacial development. International Journal of Developmental Biology, 49, 443–448.
Persons, W. S, I. V., & Currie, P. J. (2011). Dinosaur Speed Demon: The caudal musculature of Carnotaurus sastrei and implications for the evolution of South American Abelisaurids. PLoS One, 6(10), e25763.
Pol, D., & Rauhut, O. W. M. (2012). A Middle Jurassic abelisaurid from Patagonia and the early diversification of theropod dinosaurs. Proceedings of the Royal Society B, 279, 3170–3175.
Pratihar, S., Nath, R. P., & Kundu, J. K. (2010). Hox genes and its role in animal development. International Journal of Science and Nature, 1(2), 101–103.
Quinlan, E. D. (2007). Anatomy and function of digit III of the Tyrannosaurus rex manus. Geological Survey of America Abstracts with Programs, 39(6), 77.
Rallis, C., Bruneau, B. G., Del Buono, J., Seidman, C. E., Seidman, J. G., Nissim, S., et al. (2003). Txb5 is required for forelimb bud formation and continued outgrowth. Development, 130, 2741–2751.
Rauhut, O. W. M. (2012). A reappraisal of a putative record of abelisauroid theropod dinosaur from the Middle Jurassic of England. Proceedings of the Geologists’ Association, 123(5), 779–786.
Richardson, M. K., Gobes, S. M. H., Van Leeuwen, A. C., Polman, J. A. E., Pieau, C., & Sánchez-Villagra, M. R. (2009). Heterochrony in limb evolution: Developmental mechanisms and natural selection. Journal of experimental zoology (Mol Dev Evol), 312B, 639–664.
Rieppel, O. (2001). Turtles as hopeful monsters. BioEssays, 23(11), 987–991.
Rodriguez-Niedenführ, M. (2011). Control of the development of limb musculature. European Journal of Anatomy, 1, 3–9.
Rostand, J. (1964). Etienne Geoffroy Saint-Hilaire et la tératogénèse expérimentale. Revue d’Histoire des Sciences, 17(1), 41–50.
Ruiz, J., Torices, A., Serrano, H., & López, V. (2011). The hand structure of Carnotaurus sastrei (Theropoda, Abelisauridae): Implications for hand diversity and evolution in abelisaurids. Palaeontology, 54(6), 1271–1277.
Russell, D. A. (1970). Tyrannosaurs from the Late Cretaceous of western Canada. National Museum of Natural Sciences, Publications in Paleontology, 1, 1–34.
Sagai, T., Hosoya, M., Mizushina, Y., Tamura, S., & Shiroishi, T. (2004). Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development, 132, 787–803.
Sampson, S. D., & Witmer, L. M. (2007). Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. Memoir of the Society of Vertebrate Paleontology, Memoir 8, 27(supplement to no 2), 32–102.
Sampson, S. D., Carrano, M. T., & Forster, C. A. (2001). A bizarre predatory dinosaur from the Late Cretaceous of Madagascar. Nature, 409, 504–506.
Sanders, R. K., & Smith, D. K. (2005). The endocranium of the theropod dinosaur Ceratosaurus studied with computed tomography. Acta Palaeontologica Polpnica, 50(3), 601–616.
Savaliev, S. V., & Alifanov, V. R. (2007). A new stdy of the brain of the predatory dinosaur Tarbosaurus bataar (Theropoda, Tyrannosauridae). Paleontological Journal, 41(3), 282–289.
Schierhorn, H. (1984). Johann Friedrich Meckel, Jr. as founder of scientific teratology. Gegenbaurs Morphologisches Jahrbuch, 130(3), 399–439.
Schwabe, G. C., Tinschert, S., Buschow, C., Meinecke, P., Wolff, G., Gillesen-Kaesbach, G., et al. (2000). Distinct mutations in the receptor tyrosine kinase gene ROR2 cause brachydactyly type B. American Journal of Human Genetics, 67, 822–831.
Schwartz, N. B., & Domowicz, M. (2002). Chondrodysplasias due to proteoglycan defect. Glycobiology, 12(4), 57–68.
Senter, P. (2010). Vestigial skeletal structures in dinosaurs. Journal of Zoology, 280, 60–71.
Senter, P., & Parrish, J. M. (2006). Forelimb function in the theropod dinosaur Carnotaurus sastrei, and its behavioral implications. PaleoBios, 26(3), 7–17.
Sereno, P. C., Beck, A. L., Dutheil, D. B., Gado, B., Larsson, H. C. E., Lyon, G. H., et al. (1998). A long-snouted predatory dinosaur from Africa and the evolution of spinosaurids. Science, 282, 1298–1302.
Sereno, P. C., & Brusatte, S. L. (2009). Comparative assessment of Tyrannosaurid interrelationships. Journal of Systematic Palaeontology, 7(4), 455–470.
Sereno, P. C., Wilson, J. A., & Conrad, J. L. (2004). New dinosaurs link southern landmasses in the Mid-Cretaceous. Proceedings of the Royal Society of London B, 271(1546), 1325–1330.
Sereno, P. C., Tan, L., Brusatte, S. L., Kriegstein, H. J., Zhao, X., & Cloward, K. (2009). Tyrannosaurid skeletal design first evolved at small body size. Science, 326, 418–422.
Settle, S. H, Jr, Rountree, R. B., Sinha, A., Thackler, A., Higgins, K., & Kingsley, D. M. (2003). Multiple joints and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Developmental Biology, 254, 116–130.
Shum, L., Coleman, C. M., Hatakeyama, Y., & Tuan, R. S. (2003). Morphogenesis and dysmorphogenesis of the appendicular skeleton. Birth defects research Part C, 69, 102–122.
Snivelly, E., Henderson, D. M., & Phillips, D. S. (2006). Fused and vaulted nasals of tyrannosaurids dinosaurs: Implications for cranial strength and feeding mechanics. Acta Paleontologica Polonica, 51(3), 435–454.
Snively, E., & Russell, A. P. (2007a). Functional variation of neck muscles and their relation to feeding style in Tyrannosauridae and other large theropod dinosaurs. The Anatomical Record, 290, 934–957.
Snively, E., & Russell, A. P. (2007b). Craniocervical feeding dynamics in Tyrannosaurus rex. Paleobiology, 33(4), 610–638.
Swanson, A. B. (1976). A classification for congenital limb malformations. Journal of Hand Surgery (American Volume), 1(1), 8–22.
Tayel, S. M., Fawzia, M. M., Al-Nageeb, N. A., Gouda, S., Al Awadi, S. A., & Naquib, K. K. (2005). A morpho-etiological description of congenital limb anomalies. Annals of Saudi Medicine, 25(3), 219–227.
Theißen, G. (2006). The proper place of hopeful monsters in evolutionary biology. Theory in Biosciences, 124, 349–369.
Theißen, G. (2009). Saltational evolution: Hopeful monsters are here to stay. Theory in Biosciences, 128, 43–51.
Tortosa, T., Buffetaut, E., Vialle, N., Dutour, Y., Turini, E., & Cheylan, G. (2014). A new abelisaurid dinosaur from the Late Cretaceous of southern France: Palaeogeographical implications. Annales de Paléontologie, 100, 63–86.
Towers, M., Mahood, R., Yin, Y., & Tickle, C. (2008). Integration of growth and specification in the chick wing digit-patterning. Nature, 452(7189), 882–886.
Tsuhiji, T., Watabe, M., Togtbaatar, K., Tsubamoto, T., Barsbold, R., Suzuki, S., et al. (2011). Cranial osteology of a juvenile specimen of Tarbosaurus bataar (Theropoda, Tyrannosauridae) from the Nemegt formation (Upper Cretaceous) of Bugin Stav, Mongolia. Journal of Vertebrate Paleontology, 31(3), 1–21.
Valasek, P., Theis, S., DeLaurier, A., Hinits, Y., Luke, G. N., Otto, A. M., et al. (2011). Cellular and molecular investigations into the development of the pectoral girdle. Developmental Biology, 357(1), 108–116.
Vargas, A. O. (2002). La extrema reduccion del radio y ulna en la evolucion de Carnotaurus sastrei: Possible perdida de función de los genes Hoxa11 y Hoxd11. Ameghinaina 39, supplement, XVIII Jornades Argentinas de Paleontologia de Vertebrados, Resumenes p. 17.
Vargas, A. O., & Fallon, J. F. (2005). Birds have dinosaur wings: The molecular evidence. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 340B(1), 86–90.
Vargas, A. O., & Wagner, G. P. (2009). Frame-shifts of digit identity in bird evolution and Cyclopamine-treated wings. Evolution and development, 11(2), 163–169.
Vargas, A. O., Kohlsdorf, T., Fallon, J. F., VandenBrooks, J., & Wagner, G. P. (2008). The evolution of HoxD-11 expression in the bird wing: Insights from Alligator mississipiensis. PLoS One, 3(10), e3325.
Vargas, A. O., Wagner, G. P., & Gauthier, J. A. (2009). Limusaurus and bird digit identity. Nature Precedings online resource. http://precedings.nature.com/documents/3828/version/1/files/npre20093828-1.pdf.
Vasiliauskas, D., Laufer, E., & Stern, C. D. (2003). A role for hairy1 in regulating chick limb bud growth. Developmental Biology, 262, 94–106.
Vogel, A., Rodriguez, C., & Izpisua-Belmonte, J. C. (1996). Involvment of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Development, 122, 1737–1750.
Vogt, T. F., & Duboule, D. (1999). Antagonists go out on a limb. Cell, 99, 563–566.
Wagner, G. P., & Gauthier, J. A. (1999). 1, 2, 3 = 2, 3, 4: A solution to the problem of the homology of the digits in the avian hand. Proceedings of the National Academy of Sciences of United States of America, 96, 5111–5116.
Williams, S. (2011). A new subadult Tyrannosaurus rex and a reassessment of ontogenetic and phylogenetic changes in Tyrannosauroid forelimb proportions. Geological Society of America Abstracts with Programs, 43(1), 120.
Wilson, J. A., Sereno, P. C., Srivastava, S., Bhatt, D. K., Khosla, A., & Sahni, A. (2003). A new Abelisaurid (Dinosauria, Theropoda) from the Lameta formation (Cretaceous, Maastrichtian) of India. Contribution from the museum of paleontology, the Unviversity of Michigan, 31(1), 1–42.
Witmer, L. M., & Ridgely, R. C. (2009). New insights into the brain, braincase and ear region of Tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. The Anatomical Record, 292, 1266–1296.
Woods, C. G., Stricker, S., Seemann, P., Stern, R., Cox, J., Sherridan, E., et al. (2006). Mutations in WNT7A cause a range of limb malformations, including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. The American Journal of Human Genetics, 79(2), 401–408.
Xu, X., Norell, M. A., Kuang, X., Wang, X., Zhao, Q., & Jia, C. (2004). Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature, 431, 680–684.
Xu, X., Clark, J. M., Forster, C. A., Norell, M. A., Erickson, G. M., Eberth, D. A., et al. (2006). A basal tyrannosauroid dinosaur from the Late Jurassic of China. Nature, 439, 715–718.
Xu, X., Clark, J. M., Mo, J., Choiniere, J., Forster, C. A., Erikson, G. M., et al. (2009). A Jurassic ceratosaur from China helps clarify avian digital homologies. Nature, 459, 940–944.
Yang, Y., & Kozin, S. H. (2009). Cell signaling regulation of vertebrate limb growth and patterning. The Journal of Bone and Joint Surgery, 91(Supplement 4), 76–80.
Young, N. M., Wagner, G. P., & Hallgrimsson, B. (2010). Development and evolvability of human limbs. Proceedings of the National Academy of Sciences of United States of America, 107(8), 3400–3405.
Zákány, J., & Duboule, D. (2007). The role of Hox genes during vertebrate limb development. Current Opinion in Genetics & Development, 17, 359–366.
Zákány, J., Fromental-Romain, C., Warot, X., & Duboule, D. (1997). Regulation of number and size of digits by posterior Hox genes: A dose-dependent mechanism with potential evolutionary implications. Proceedings of the National Academy of Sciences of United States of America, 94, 13695–13700.
Zeller, R., López-Rios, J., & Zuniga, A. (2009). Vertebrate limb bud development: Moving towards integrative analysis of organogenesis. Nature Reviews Genetics, 10, 845–858.
Zhang, Z., Verheyden, J. M., Hassell, J. A., & Sun, X. (2009). FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Developmental Cell, 16, 607–613.
Zuniga, A., Zeller, R., & Probst, S. (2012). The molecular basis of human congenital limb anomalies. Wiley Interdisciplinary Reviews: Developmental Biology, 1(6), 803–822.
Acknowledgments
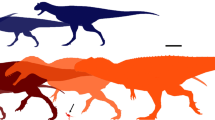
Didier Marchand for commenting the first draft regarding a concept which was always discreetly around us when we discussed evolutionary concepts. Remi Laffont, Stephen Brusatte, Phil Senter, Christopher A. Brochu, Mohammad Al-Qattan, Rolf Zeller and Ariana P. Carabajal for their help in providing some references. Two anonymous reviewers and editor Benedikt Hallgrimsson for useful comments regarding specific parts and length reduction. R. Guinard for checking the manuscript. Age ranges of taxa is an information provided by fossilworks.org, as well as complementary measurements of specimens. Silhouettes used for Fig. 6 are provided by phylopic.org (credits: Scott Hartman, Matt Martyniuk, T. Michael Keesey, Craig Dylke, Stephen O’Connor, Robert Gay, Michael B. H and Emily Willoughby).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guinard, G. Introduction to Evolutionary Teratology, with an Application to the Forelimbs of Tyrannosauridae and Carnotaurinae (Dinosauria: Theropoda). Evol Biol 42, 20–41 (2015). https://doi.org/10.1007/s11692-014-9296-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-014-9296-1