Abstract
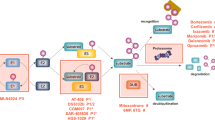
Ubiquitin-proteasome pathway mediates the degradation of cell protein, and cell cycle, gene translation and expression, antigen presentation and inflammatory development. Proteasome inhibitor can inhibit growth and proliferation of tumor cell, induce apoptosis and reverse multipledrug resistance of tumor cell, increase the sensitivity of other chemotherapeutic drugs and radiotherapy, and is a novel class of potent anti-tumor agents.
Similar content being viewed by others
References
Voorhees PM, Dees EC, O’Neil B, et al. The proteasome as a target for cancer therapy[J]. Clin Cancer Res 2003; 9:6316–6325.
Tao GZ, Rott LS, Lowe AW, et al. Hyposmotic stress induces cell growth arrest via proteasome activation and cyclin/cyclin-dependent kinase degradation[J]. J Biol Chem 2002; 277: 19295–19303.
Xu J, Attisano L. Mutations in the tumor suppressors Smad2 and Smad4 inactivate transforming growth factor beta signaling by targeting smads to ubiquitin-proteasome pathway[J]. Proc Natl Acad Sci USA 2000; 97: 4820–4825.
Ogiso Y, Tomida A, Kim HD, et al. Glucose starvation and Hypoxia induce nuclear accumulation of proteasome in cancer cells[J]. Biochem Biophys Res Commun 1999; 258: 448–452.
Aghajanian C, Soigent S, Dizon DS, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies[J]. Clin Cancer Res 2002; 8: 2505–2511.
Sakata N, Dixon JL. Ubiquitin-proteasome-dependent degradation of apolipoprotein B100 in vitro[J]. Biochem Biophys Acta 1999; 1437: 71–79.
Koguchi Y, Kohno J, Nishio M, et al. Tmc-95A, B, C, and D, novel proteasome inhibitors produced by Apiospora montagnei Sacc. TC1093. Taxonomy, production, isolation, and biological activities[J]. J Antibiot 2000; 53: 105–109.
Kazi A, Daniel KG, Smith DM, et al. Inhibition of proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein[J]. Biochem Pharmacol 2003; 66: 965–976.
Adams J. Development of the proteasome inhibitor PS-341[J]. Oncologist 2002; 7: 9–16.
Leblanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model[J]. Cancer Res 2002; 62: 4996–5000.
Mitchell BS. The proteasome-an emerging therapeutic target in cancer[J]. N Engl J Med 2003; 348: 2597–2598.
Hochwald SN, Lind DS, Malaty J, et al. Antincoplastic therapy in colorectal cancer through proteasome inhibition[J]. Am Surg 2003; 69:15–23.
Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibtiors: a novel class of potent and effective antitumor agents[J]. Cancer Res 1999; 59: 2615–2622.
Cusack JC Jr, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341:Implication for systemic nuclear factor-KB inhibitor[J]. Cancer Res 2001; 61: 3535–3540.
Fan XM, Wong BC, Wang WP, et al. Inhibition of proteasome function induced apoptosis in gastric cancer[J]. Int J Cancer 2001; 93: 481–488.
Chen F, Chang D, Goh M, et al. Role of p53 in cell cycle regulation and apoptosis following exposure to proteasome inhibitors[J]. Cell Growth Differ 2000; 11: 239–246.
Fukuchi K, Hagiwara T, Nakamura K, et al. Identification of the regulatory region required for ubiquitination of the cyclin kinase inhibitor, p21[J]. Biochem Biophys Res Commun 2002; 293: 120–125.
Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells[J]. Proc Natl Acad Sci USA 2002; 99: 14374–14379.
Adams J, Kauffman M. Development of the proteasome inhibitor Velcade(Bortezomib) [J]. Cancer Invest 2004; 22:304–311.
Esparis-Ogando A, Alegre A, Aguado B, et al. Bortezomib is an efficient agent in plasma cell leukemias[J]. Int J Cancer 2005; 114: 665–667.
Loo TW, Clarke DM. The human multidrug resistance P-glycoprotein is inactive when its maturation is inhibited: potential for a role in cancer chemotherapy [J]. FASEB J 1999; 13: 1724–1732.
Loo TW, Clarke DM. Superfolding of the partially unfolded Core-glycosylated intermediate of human P-glycoprotein into the mature enzyme is promoted by substrate-induced transmembrane domain interactions [J]. J Biol Chem 1998; 273: 14671–14674.
Kim OH, Lim JH, Woo KJ, et al. Inference of p53 and p21WAF1 expression on G2/M phase arrest of colorectal carcinoma HCT 116 cells to proteasome inhibitors[J]. Int J Oncol 2004; 24: 935–941.
Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells[J]. Cancer Res 2001; 61: 3071–3076.
An WG, Hwang SG, Trepel JB, et al. Protease inhibitor-induced apoptosis: accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition[J]. Leukemia 2000; 14: 1276–1283.
Frankel A, Man S, Elliott P, et al. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341[J]. Clin Cancer Res 2000; 6:3719–3728.
Yunmbam MK, Li QQ, Mimnaugh EG, et al. Effect of the proteasome inhibitor ALLnL on cisplatin sensitivity in human ovarian tumor cells[J]. Int J Oncol 2001; 19: 741–748.
Fujita T, Washio K, Takabatake D, et al. Proteasome inhibitors can alter the signaling pathways and attenuate the P-glycoprotein-mediated multidrug resistance[J]. Int J Cancer 2005; 117: 670–682.
Lin ZP, Boller YC, Amer SM, et al. Prevention of brefeldin A-induced resistance to teniposide by the proteasome inhibitor MG-132: involvement of NF-κB activation in drug resistance[J]. Cancer Res 1998; 58: 3059–3065.
Pahl HL, Baeuerle PA. The ER-overload response: activation of NF-κB[J]. Trends Biochem Sci 1997; 22: 63–67.
Denlinger CE, Rundall BK, Keller MD, et al. Proteasome inhibition sensitizes non-small-cell lung cancer to gemcitabine-induced apoptosis[J]. Ann Thorac Surg 2004; 78: 1207–1214.
Orlowski RZ, Voorhees PM, Garcia RA, et al. Phase I trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies[J]. Blood 2005; 105:3058–3065.
Richardson PG, Barlogie B, Berenson J, et al. A phase II study of bortezomib in relapsed, refractory myeloma[J]. N Engl J Med 2003; 348: 2609–2617.
Richardson PG, Barlogie B, Berenson J, et al. Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma: final time-to-event results from the SUMMIT trial[J]. Cancer 2006; 106:1316–1319.
Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Treatment of relapsed or refractory Waldenstrom’s macroglobulinemia with bortezomib [J]. Haematologica 2005; 90: 1655–1658.
Kondagunta GV, Drucker B, Schwartz L, et al. Phase II clinical study of bortezomib for patients with advanced renal cell carcinoma[J]. J Clin Oncol 2004; 22: 3720–3725.
Alberts SR, Foster NR, Morton RF, Kugler J, Schaefer P, Wiesenfeld M, Fitch TR, Steen P, Kim GP, Gill S. PS-341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group (NCCTG) randomized phase II study[J]. Ann Oncol 2005; 16: 1654–1661.
Ciolli S, Leoni F, Gigli F, et al. Low dose velcade, thalidomide and dexamethasone (LD-VTD): An effective regimen for relapsed and refractory multiple myeloma patients[J]. Leuk Lymphoma 2006; 47: 171–173.
Berenson JR, Yang HH, Sadler K, et al. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma[J]. J Clin Oncol 2006; 24: 937–944.
Hollmig K, Stove J, Talamo G, et al. Addition of bortezomib(Velcade) to high dose melphalan (Vel-Mel) as an effective conditioning regimen with autologous stem cell support in multiple myeloma (MM)[J]. Blood 2004; 104: 266a.
Harousseau JL, Attal M, Leleu X, et al. Bortezomib (Velcade) plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: Preliminary results of an IFM phase II study[J]. Blood 2004; 104: 416a.
Rosinol L, Montoto S, Ciberira MT, et al. Bortezomib-induced severe hepatitis in multiple myeloma: a case report[J]. Arch Intern Med 2005; 165: 464–465.
Engelhardt M, Muller AM, Maier W, et al. Severe irreversible bilateral hearing loss after Bortezomib (Velcade) therapy in a multiple myeloma(MM) patient[J]. Leukemia 2005; 19: 869–870.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Outstanding Youth Scientists Foundation of china (No. 30225038) and the Youth and Middle-Age Scientists Science and Research Found of the Affiliated Hospital, Wuhan University of Science and Technology.
Rights and permissions
About this article
Cite this article
Zhou, Ym., Yu, Mx., Long, H. et al. Anti-tumor action and clinical application of proteasome inhibitor. Chin. J. Cancer Res. 20, 77–84 (2008). https://doi.org/10.1007/s11670-008-0077-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11670-008-0077-1