Abstract
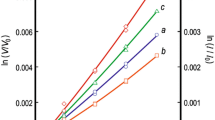
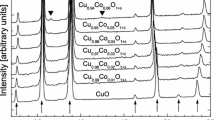
There are conflicting reports in the literature regarding solid solubility in the system RuO2-TiO2. To resolve this issue a few experiments were conducted in air at 1673, 1723, and 1773 K. The results show limited terminal solid solubility. There is an extended solid-state miscibility gap that intersects the decomposition curve for the RuO2-rich solid solution generating a peritectoid reaction at 1698 K. The measured equilibrium compositions of the solid solutions are used to develop a thermodynamic description of the oxide solid solution with rutile structure. Using the subregular solution model, the enthalpy of mixing can be represented by the expression, \( \Delta H^{\rm M} /{\text{J}} \cdot {\text{mol}}^{{ - 1}} = X_{{{\text{TiO}}_{2} }} X_{{{\text{RuO}}_{2} }} {\left( {34,100X_{{{\text{TiO}}_{2} }} + 30,750X_{{{\text{RuO}}_{2} }} } \right)} \). The binodal and spinodal curves and T-X phase diagram in air are computed using this datum and Gibbs energy of formation of RuO2 available in the literature. The computed results suggest that equilibrium was not attained during solubility measurements at lower temperatures reported in the literature.



Similar content being viewed by others
References
J. Qu, X. Zhang, Y. Wang, C. Xie, Electrochemical Reduction of CO2 on RuO2-TiO2 Nanotube Composite Modified Pt Electrode, Electrochim. Acta, 2005, 50(16-17), p 3576-3580
C. Comninellis, G.P. Vercesi, Characterization of DSA-Type Oxygen Evolving Electrodes: Choice of a Coating, J. Appl. Electrochem., 1991, 21, p 335-345
K.T. Jacob, S. Mishra, Y. Waseda, Refinement of Thermodynamic Properties of Ruthenium Dioxide and Osmium Dioxide, J. Am. Ceram. Soc., 2000, 83(7), p1745-1752
R.D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Inter-atomic Distances in Halides and Chalcogenides, Acta Crystallogr., 1976, A32, p 751
M. Hrovat, J. Holc, D. Kolar, Phase Equilibria in the RuO2-TiO2-Al2O3 and RuO2-TiO2-Bi2O3 Systems, J. Mater. Sci. Lett., 1993, 12, p 1858-1860
M. Hrovat, J. Holc, Z. Samardzija, G. Drazic, The Extent of Solid Solubility in the RuO2-TiO2 System, J. Mater. Res., 1996, 11(3), p 727-732
H.K. Hardy, A Sub-Regular Solution Model and Its Application to Some Binary Alloy Systems, Acta Metall., 1953, 1, p 202-209
M.W. Chase Jr., C.A. Davies, J.R. Downey Jr., D.J. Frurip, R.A. McDonald, A.N. Syverud, JANAF Thermochemical Tables, 3rd ed, J. Phys. Chem. Ref. Data, 1985, 14 (suppl. 1), p 1681
Acknowledgment
One of the authors, R. Subramanian, is grateful to the Indian Institute of Science, Bangalore, for the award of Young Engineering Fellowship, which facilitated this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacob, K., Subramanian, R. Phase Diagram for the System RuO2-TiO2 in Air. J Phs Eqil and Diff 29, 136–140 (2008). https://doi.org/10.1007/s11669-007-9236-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-007-9236-1