Abstract
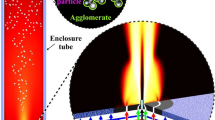
The development of a new quenching design combining rapid cooling with an expansion for controlling the size of nanoparticles synthesized at industrial scale by flame spray pyrolysis was investigated. The design of the quenching device was supported by simulations using a coupled computational fluid dynamics-monodisperse aerosol model to reduce the size of the primary particles and their agglomerate diameters while conserving the production yield at the filter above the burner. The results showed that quenching the spray flame in an open environment led to lower production yield due to the negative velocity of quenching gas which diverted the particles to the bottom of reactor. An additional upstream air flow could help to increase the particle production yield at high air flow rates, while it had a negative effect on the penetration depth of quenching gas inside the main flame which resulted in higher flame heights. The new design showed that adding an enclosure around the burner and quenching ring can significantly increase the quenching efficiency and reduce the particle size. The technique to control the particle size was also studied in this paper.















Similar content being viewed by others
Abbreviations
- a :
-
Area of agglomerate particle, m2
- a s :
-
Area of a completely fused (spherical) aggregate of volume v a
- A :
-
Dimensionless coefficient in eddy dissipation model
- A d :
-
Surface area of the droplet, m2
- B :
-
Dissipation term
- c :
-
Particle velocity, m/s
- c p :
-
specific heat capacity, J/(kg K)
- C :
-
Scaling constant
- C D :
-
Drag coefficient
- C i,s :
-
Concentration of component i on the droplet surface
- C i,∞ :
-
Concentration of component i in the bulk
- d :
-
Droplet diameter, m
- d o :
-
Orifice diameter, m
- d i :
-
Initial droplet size, m
- D :
-
Particle diffusion coefficient, m2/s
- D i,m :
-
Diffusion coefficient of vapor in the bulk, m2/s
- D dg :
-
Droplet to gas density ratio
- D ω :
-
Cross diffusion term
- e * :
-
Sensible enthalpy, J/kg
- E :
-
Total enthalpy, J
- f :
-
Stoichiometric mass requirement of oxidant to consume 1 kg of fuel
- F :
-
Force, N
- g :
-
Gravitational acceleration, m2/s
- g t :
-
Gas transition parameter, m
- \(\tilde{G}_{k}\) :
-
Generation of k
- G ω :
-
Generation of ω
- g :
-
Gravitational acceleration, m2/s;
- h :
-
Convective heat transfer coefficient, W/(m2 K)
- h v,i :
-
Latent heat of vaporization, J/kg
- H :
-
Dimensionless coefficient in eddy dissipation model
- I :
-
Radiation intensity
- J :
-
Diffusion flux
- k :
-
Kinetic energy, m2/s2
- k b :
-
Boltzmann constant, J/K
- K :
-
Thermal conductivity, W/(m/K)
- Kn :
-
Knudsen number
- m :
-
Mass, kg
- \(\dot{m}\) :
-
Mass flow rate/vaporization rate, kg/s
- M w :
-
Molecular weight, g/mol
- n :
-
Refractive index
- N :
-
Number concentration of the particles per unit mass of gas, #particles/kggas
- p :
-
Pressure, Pa
- Pr :
-
Prandtl number
- r c :
-
Collision radius of an aggregate, m
- r p :
-
Radius of primary particle, m
- R :
-
Universal gas constant, J/(mol K)
- R i :
-
Reaction rate
- Re :
-
Reynolds number
- Re d :
-
Reynolds number based on droplet diameter
- \(\vec{s}^{\prime}\) :
-
Scattering direction vector
- S :
-
Source term
- Sc :
-
Schmidt number
- t :
-
Time, s
- T :
-
Temperature, K
- v :
-
Velocity, m/s
- v a :
-
Volume of agglomerate particle, m3
- We :
-
Weber number
- x :
-
Mole fraction
- Y :
-
Mass fraction
- z :
-
Absorption coefficient, m−1
- β:
-
Collision kernel between agglomerates, m3/s
- γ:
-
Surface tension, N/m
- ɛd :
-
Droplet emissivity
- μ:
-
Dynamic viscosity, kg/(m s)
- ρ:
-
density, kg/m3
- σ:
-
Stefan-Boltzmann constant, W/(m2 K4)
- σs :
-
Scattering coefficient, m−1
- τ:
-
Deviatoric stress tensor
- ω:
-
Specific dissipation rate, s−1
- Γ:
-
Effective diffusivity
- Φ:
-
Phase function
- Ω′:
-
Solid angle
- eff:
-
Effective value in reference to the addition of turbulent and non-turbulent contribution of a variable
- d:
-
Droplet
- g :
-
Gas
- k:
-
Kinetic energy
- mix:
-
Mixture
- P:
-
Particle
- R:
-
Radiation
- sin:
-
Sintering
- ∞:
-
Continuous phase
- ω:
-
Specific dissipation rate, s−1
References
C.R. Bickmore, K.F. Waldner, R. Baranwal, T. Hinklin, D.R. Treadwell, and R.M. Laine, Ultrafine Titania by Flame Spray Pyrolysis of a Titanatrane Complex, J. Eur. Ceram. Soc., 1998, 18, p 287-297
K.A. Gross, J. Tikkanen, J. Keskinen, V. Pitkänen, M. Eerola, R. Siikamaki, and M. Rajala, Liquid Flame Spraying for Glass Coloring, J. Thermal Spray Technol., 1999, 8, p 583-589
R. Mueller, L. Mädler, and S.E. Pratsinis, Nanoparticle Synthesis at High Production Rates by Flame Spray Pyrolysis, Chem. Eng. Sci., 2003, 58, p 1969-1976
G.-J. Yang, C.-J. Li, and Y.-Y. Wang, Phase Formation of Nano-TiO2 Particles During Flame Spraying with Liquid Feedstock, J. Thermal Spray Technol., 2005, 14, p 480-486
R. Kavitha, S. Meghani, and V. Jayaram, Synthesis of Titania films by Combustion flame Spray Pyrolysis Technique and its Characterization for Photocatalysis, Mater. Sci. Eng.: B, 2007, 139, p 134-140
J.S. Lee, M.H. Oh, P. Kumar, A. Khanna, R.K. Singh, and M.B. Ranade, Mn-Doped Zn2SiO4 Phosphors Synthesis Using Flame Spray Pyrolysis, J. Thermal Spray Technol., 2011, 20, p 1001-1008
H. Torabmostaedi, T. Zhang, P. Foot, S. Dembele, and C. Fernandez, Process Control for the Synthesis of ZrO2 Nanoparticles Using FSP at High Production Rate, Powder Technol., 2013, 246, p 419-433
H. Torabmostaedi and T. Zhang, Computational Study of the Effect of Processing Parameters on the Formation and Growth of ZrO2 Nanoparticles in FSP Process, Chem. Eng. Process., 2014, 78, p 1-10
D. Noriler, C.D. Rosebrock, L. Mädler, H.F. Meier, and U. Fritsching, Influence of Atomization and Spray Parameters on the Flame Spray Process for Nanoparticle Production, At. Sprays, 2014, 24, p 495-524
L. Mädler, H.K. Kammler, R. Mueller, and S.E. Pratsinis, Controlled Synthesis of Nanostructured Particles by Flame Spray Pyrolysis, J. Aerosol. Sci., 2002, 33, p 369-389
D.S. Jung, S.K. Hong, J.S. Cho, and Y.C. Kang, Nano-sized Barium Titanate Powders with Tetragonal Crystal Structure Prepared by flame Spray Pyrolysis, J. Eur. Ceram. Soc., 2008, 28, p 109-115
A.I.Y. Tok, F.Y.C. Boey, S.W. Du, and B.K. Wong, Flame Spray Synthesis of ZrO2 Nano-particles Using Liquid Precursors, Mater. Sci. Eng. B, 2006, 130, p 114-119
H. Torabmostaedi and T. Zhang, Effect of Nozzle Geometry and Processing Parameters on the Formation of Nanoparticles Using FSP, Eng. Res. Des, Chem, 2014, doi:10.1016/j.cherd.2014.01.011
H. Schulz, L. Mädler, R. Srobel, R. Jossen, S.E. Pratsinis, and T. Johannessen, Independent Control of Metal Cluster and Ceramic Particle Characteristics During One-Step Synthesis of Pt/TiO2, J. Mater. Res., 2005, 20, p 2568-2577
J.P. Hansen, J.R. Jensen, H. Livbjerg, and T. Johannessen, Synthesis of ZnO Particles in a Quench-Cooled Flame Reactor, AIChE J., 2001, 47, p 2413-2418
K. Wegner, S. Vinati, P. Piseri, A. Antonini, A. Zelioli, E. Barborini, C. Ducati, and P. Milani, High-Rate Production of Functional Nanostructured Films and Devices by Coupling Flame Spray Pyrolysis with Supersonic Expansion, Nanotechnology, 2012, 23, p 185603
K. Wegner, W.J. Stark, and S.E. Pratsinis, Flame-Nozzle Synthesis of Nanoparticles with Closely Controlled Size, Morphology and Crystallinity, Mater. Lett., 2002, 55, p 318-321
R. Mueller, R. Jossen, H.K. Kammler, S.E. Pratsinis, and M.K. Akhtar, Growth of Zirconia Particles Made by Flame Spray Pyrolysis, AIChE J., 2004, 50, p 3085-3094
R. Mueller, R. Jossen, S.E. Pratsinis, M. Watson, and M.K. Akhtar, Zirconia Nanoparticles Made in Spray Flames at High Production Rates, J. Am. Ceram. Soc., 2004, 87, p 197-202
S. Chandrasekhar, Radiative Transfer, Dover Publications, New York, 1960
F.R. Menter, Two-Equation Eddy-Viscosity Turbulence Models for Engineering Applications, AIAA J., 1994, 32, p 1598-1605
B.F. Magnussen and B.H. Hjertager, On Mathematical Modeling of Turbulent Combustion with Special Emphasis on Soot Formation and Combustion, Symp. (Int.) Combust., 1977, 16, p 719-729
S.A. Morsi and A.J. Alexander, An Investigation of Particle Trajectories in Two-Phase Flow Systems, J. Fluid Mech., 1972, 55, p 193-208
P. Hutchinson, G.F. Hewitt, and A.E. Dukler, Deposition of Liquid or Solid Dispersions from Turbulent Gas Streams: A Stochastic Model, Chem. Eng. Sci., 1971, 26, p 419-439
G.A. Kallio and M.W. Reeks, A Numerical Simulation of Particle Deposition in Turbulent Boundary Layers, Int. J. Multiphase Flow, 1989, 15, p 433-446
M.M. Elkotb, M.A. Madhy, and M.E. Montaser, Investigation of External-Mixing Airblast Atomizers, Proceedings of the 2nd International Conference on Liquid Atomization and Sprays, Madison, 1982
N.A. Fuchs, Evaporation and Droplet Growth in Gaseous Media, Pergamon Press, London, 1959, p 1959
S.S. Sazhin, Advanced Models of Fuel Droplet Heating and Evaporation, Prog. Energy Combust. Sci., 2006, 32, p 162-214
J.P. Van Doormaal and G.D. Raithby, Enhancements of the SIMPLE Method for Predicting Incompressible Fluid Flows, Numer. Heat. Transfer., 1984, 7, p 147-163
F.E. Kruis, K.A. Kusters, S.E. Pratsinis, and B. Scarlett, A Simple Model for the Evolution of the Characteristics of Aggregate Particles Undergoing Coagulation and Sintering, Aerosol Sci. Technol., 1993, 19, p 514-526
W. Koch and S.K. Friedlander, The Effect of Particle Coalescence on the Surface Area of a Coagulating Aerosol, J. Colloid Interface Sci., 1990, 1990, p 419-427
J.H. Seinfeld, Atmospheric Chemistry and Physics of Air Pollution, Wiley, New York, 1986
A. Kobata, K. Kusakabe, and S. Morooka, Growth and Transformation of TiO2 Crystallites in Aerosol Reactor, AIChE J., 1991, 37, p 347-359
U. Brossmann, R. Würschum, U. Södervall, and H.E. Schaefer, Oxygen Diffusion in Ultrafine Grained Monoclinic ZrO2, J. Appl. Phys., 1999, 85, p 7646-7654
A. Madeyski and W.W. Smeltzer, Oxygen Diffusion in Monoclinic Zirconia, Mater. Res. Bull., 1968, 3, p 369-375
M. Rösner-Kuhn, W.H. Hofmeister, G. Kuppermann, R.J. Bayuzick, and M.G. Frohberg, Investigations of the Influence of Oxygen on the Surface Tension of Zirconium by the Oscillating Drop Technique, Surf. Sci., 1999, 443, p 159-164
T. Seto, A. Hirota, T. Fujimoto, M. Shimada, and K. Okuyama, Sintering of Polydisperse Nanometer-Sized Agglomerates, Aerosol Sci. Technol., 1997, 27, p 422-438
Acknowledgments
This work was supported by the European Community’s Seventh Framework Program under Grant No. CP-FP 228885-2.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Model Validation
The accuracy of the 3D model developed in this work (quenching device incorporated in the mesh but no quenching gas applied) for prediction of the flame height and particle size was examined by comparing the simulated results with those calculated in our previous work (Ref 7) and measured by Mueller et al. (Ref 19). Figure 16 shows the flame heights of three simulated flames at feed rates of 6.8, 54.1, and 81.1 mL/min using 1 M ZnP concentration in ethanol. The dispersion gas flow rate and its pressure drop were kept constant at 50 L/min and 1 bar, respectively, for all precursor feed rates. Increasing the solution feed rate increased the supplied fuel energy to the flame which resulted in higher flame height. Comparing the flame heights, good agreement was found between the 3D model (this work), the 2D model,7 and the measured values obtained by Mueller et al. (Ref 19).
Figure 17 shows the comparison between numerical (3D and 2D model) and measured (Ref 19) data for BET-equivalent average diameter of the zirconia primary particles as a function of production rate at 1 M precursor concentration. The results from the 3D model were in good agreement with the previously calculated (Ref 7) and the measured (Ref 19) results. Increasing the production rate from 50 to 300 g/h increased the primary zirconia particle diameter from 6.7 to 19.4 nm (solution feed rates were from 6.8 to 81.1 mL/min using 1 M ZnP in ethanol, which were dispersed by 50 L/min oxygen at 1 bar pressure drop), while the measured values were 6 to 19 nm, respectively. The validated 3D model was then used to optimize the quenching efficiency in FSP.
Rights and permissions
About this article
Cite this article
Torabmostaedi, H., Zhang, T. Numerical Optimization of Quenching Efficiency and Particle Size Control in Flame Synthesis of ZrO2 Nanoparticles. J Therm Spray Tech 23, 1478–1492 (2014). https://doi.org/10.1007/s11666-014-0148-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-014-0148-4