Abstract
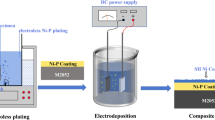
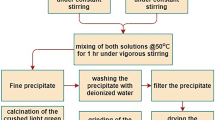
In the present study, Ni-P-ZnO nanocomposite coating was developed on the surface of mild steel substrate by electroless technique. The second phase ZnO nanoparticles were synthesized by autocombustion method, to incorporate into the Ni-P matrix. 10 g/L of ZnO nanoparticles were added to the Ni-P alkaline bath for co-deposition, and the electroless bath was reduced by sodium hypophosphite. The heat treatment of the as-prepared Ni-P/Ni-P-ZnO coatings was carried out at 400 °C in argon (99.9%) atmosphere for 1 h. The as-prepared and heat-treated Ni-P/Ni-P-ZnO coatings and ZnO nanoparticles were analyzed for surface morphology, elemental composition, phase analysis and particle size distribution using field emission scanning electron microscopy (FESEM), energy-dispersive analysis of x-rays (EDAX), transmission electron microscopy (TEM), powder x-ray diffraction analysis. To determine the calcinations temperature of ZnO powder, differential scanning calorimetry was also carried out. TEM analysis of the synthesized ZnO nanoparticles was also carried out, and from the micrographs a spherical shape of ~40 nm size range is observed for the ZnO nanoparticles. The corrosion properties of the coatings were carried out in a 3.5 wt.% NaCl solution by electrochemical polarization test. The dispersion of ZnO nanoparticles into the coating is determined by FESEM. Atomic force microscopy was used to investigate the change in the surface topography of the coatings before and after exposure in the test environment. A uniform distribution of ZnO nanoparticles into the Ni-P matrix is confirmed by the FESEM-EDAX results. Electrochemical test results suggest that Ni-P-ZnO shows better corrosion resistance as compared to plain Ni-P coating.









Similar content being viewed by others
References
A. Brenner and G. Riddell, Res. Natl. US Bur. Stand., 1946, 37, p 31
A. Brenner, D.E. Couch, and E.K. Williams, J. Res. Nat. Bur. Stand., 1950, 44, p 109
R.C. Agarwala and V. Agarwala, Sadhana, 2003, 28, p 475–493
R.C. Agarwala, Ph.D. thesis, Department of Metallurgical and Materials Engineering, University of Roorkee (presently IIT, Roorkee), (1987)
P.K. Datta, P.B. Bedingfield, D.B. Lewis and P.B. Wells, ‘Structure and phase changes accompanying treatment of electroless Ni-B alloy coating, Conf. Proc. 2nd Int. Electroless Nickel Conference Solihull, 1991, p 139–153
A. Srivastava, S. Mohan, V. Agarwala, and R.C. Agarwala, Z. Metallkd., 1992, 83, p 251–253
K.H. Krishnan, J. Praveen, M. Ganesan, P.M. Kavimani, S. John, and K.N. Srinivasan, Mater. Perform., 2006, 45, p 36–39
J.N. Balaraju and K.S. Rajam, Surf. Coat. Technol., 2005, 195, p 154–161
D.H. Kim, K. Aoki, and O. Takano, J. Electrochem. Soc., 1995, 142, p 3763–3767
L. Wang, L. Zhao, B. Zhang, S. Liao, Y. OnYang, and W. Hu, Z. Metallkd., 1997, 88, p 945–948
S.B Sharma, Ph.D. Thesis. ‘Synthesis and Tribological Characterization of Ni-P Based Electroless Composite Coatings’. IIT Roorkee, India, 2002
J. Sudagar, J. Lian, and W. Sha, J. Alloys Compd., 2013, 571, p 183–204
I. Apachitei, F.D. Tichelaar, J. Duszczyk, and L. Katgerman, Surf. Coat. Technol., 2002, 149, p 263–278
G. Jiaqiang, L. Lei, W. Yating, S. Bin, and H. Wenbin, Surf. Coat. Technol., 2006, 200, p 5836–5842
Y.S. Huang, X.T. Zeng, I. Annergren, and F.M. Liu, Surf. Coat. Technol., 2003, 167, p 207–211
D. Dong, X.H. Chen, W.T. Xiao, G.B. Yang, and P.Y. Zhang, Appl. Surf. Sci., 2009, 255, p 7051–7055
T. Rabizadeh and S.R. Allahkaram, Mater. Des., 2011, 32, p 133–138
W. Chen, W. Gao, and Y. He, Surf. Coat. Technol., 2010, 204, p 2493–2498
S.M. Moonir-Vaghefi, A. Saatchi, and J. Hejazi, Met. Finish., 1997, 95, p 46–52
M.D. Ger and B.J. Hwang, Mater. Chem. Phys., 2002, 76, p 38–45
P.R. Ebdon, Plat. Surf. Finish., 1988, 75, p 65–68
A. Ramalho and J.C. Miranda, Wear, 2005, 259, p 828–834
W.X. Chen, J.P. Tu, Z.D. Xu, R. Tenne, R. Rosenstveig, W.L. Chen, and H.Y. Gan, Adv. Eng. Mater., 2002, 4, p 686–690
O.A. Leon, M.H. Staia, and H.E. Hintermann, Surf. Coat. Technol., 2005, 200, p 1825–1829
N. Feldstein, G.O. Mallory and J.B. Hajdu, ‘Electroless Plating: Fundamentals and Applications’, American Electroplaters and Surface Finishers Society, Orlando, Florida,(Eds.), 1990, p 269.
S. Sharma, C.K. Saini, S. Sharma, and V. Agarwala, J. Mater. Environ. Sci., 2014, 5(5), p 1667–1670
S. Sharma, S. Sharma, P. Agarwala, R. Garg, and P. Gopinath, Adv. Mater. Res., 2012, 585, p 512–516
T. Rabizadeh, S.R. Allahkaram, and A. Zarebidaki, Mater. Des., 2010, 31, p 3174–3179
P.K. Roy, J. Mater. Sci. Res., 2012, 1, p 28–34
A. Zoikis-Karathanasis, E.A. Pavlatou, and N. Spyrellis, Electrochim. Acta, 2009, 54, p 2563–2570
E. Valova, I. Georgiev, S. Armyanov, J.L. Delplancke, B. Tachev, T. Tsa-Cheva, and J. Dille, J. Electrochem. Soc., 2001, 148(4), p 266
M. Bouanani, F. Cherkaoui, R. Fratesi, G. Roventi, and G. Barucca, J. Appl. Electrochem., 1999, 29, p 637
B. Veeraraghavan, B. Haran, S.P. Kumaraguru, and B. Popov, J. Electrochem. Soc., 2003, 150(4), p B131–B139
K. Zielinska, A. Stankiewicz, and I. Szczygiel, J. Colloid Interface Sci., 2012, 377, p 362–367
J.N. Balaraju, C. Anandan, and K.S. Rajam, Appl. Surf. Sci., 2005, 250, p 88–97
J.N. Balaraju, T.S.N. Sankara Narayanan and S.K. Seshadri, J. Appl. Electrochem., 2003, 33, p 807–816
S.R. Allahkaram, R. Faezi Alivand, and M.S. Bakhsh, Iran. J. Mater. Sci. Eng., 2013, 10, p 11–17
S.M.A. Shibli, B. Jabeera, and R.I. Anupama, Surf. Coat. Technol., 2006, 200, p 3903
S.M.A. Shibli, B. Jabeera, and R.I. Anupama, Appl. Surf. Sci., 2006, 253, p 1644–1648
Sarika, Ph.D. Thesis, Development of Electroless Ni-P-ZnO Nanocomposite Coatings, Graphic Era University, 2015
A.S. Hamdy, M.A. Shoeib, H. Hady, and O.F. Abdel, Salam, Surf. Coat. Technol., 2007, 202, p 162–171
A. Babanejhad, M. Hashemi, Y. Rahmatallahpur, and ShA Nozad, Bull. Mater. Sci., 2012, 35, p 561–566
Acknowledgments
Authors acknowledge Uttarakhand State Biotechnology Department (USBD), Haldwani, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S., Sharma, S., Sharma, A. et al. Co-deposition of Synthesized ZnO Nanoparticles into Ni-P Matrix Using Electroless Technique and Their Corrosion Study. J. of Materi Eng and Perform 25, 4383–4393 (2016). https://doi.org/10.1007/s11665-016-2292-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-016-2292-0