Abstract
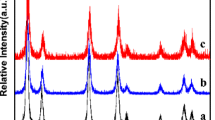
We have investigated the influence of Ni doping on the physicochemical properties of CeO2 synthesized by a co-precipitation process. As-prepared nanoparticles were characterized by x-ray diffraction pattern (XRD), transmission electron microscopy (TEM), energy dispersive x-ray analysis, thermal analysis, Fourier transform infrared spectra, optical absorption and temperature program reduction techniques. The observed results clearly demonstrate the impact of Ni ion concentration on the crystallinity, optoelectronic and reducibility of CeO2 nanoparticles. XRD results show that the particle size was decreased after increasing the Ni ion-doping concentrations. TEM micrographs exhibited high aggregation in high Ni ion-doping concentration causing the smallest grain size of the materials. The band gap energies increased with decreasing particle size because of the higher oxygen-releasing capacity and stronger interaction between nickel and the CeO2 matrix. The 7% mol Ni-doped CeO2 exhibits low-temperature reduction. Because of excellent optoelectronic and redox properties, magnetically active Ni ion-doped CeO2 nanoparticles can be used for electrochemical biosensors and solid oxide fuel cell catalysts can be potentially extended to other applications.
Similar content being viewed by others
References
R.W. Tarnuzzer, J. Colon, S. Patil, and S. Seal, Nano Lett. 5, 2573 (2005).
S. Patil, S. Seal, Y. Guo, A. Schulte, and J. Norwood, Appl. Phys. Lett. 88, 243110 (2006).
F. Zhang, S.W. Chan, J.E. Spanier, E. Apak, Q. Jin, R.D. Robinson, and I.P. Herman, Appl. Phys. Lett. 80, 127 (2002).
C.G. Levi, J.Y. Yang, B.J. Dalgleish, F.W. Zok, and A.G. Evans, J. Am. Ceram. Soc. 81, 2077 (1998).
S. Tsunekawa, S. Ito, and Y. Kawazoe, Appl. Phys. Lett. 85, 3845 (2004).
S. Patil, S.C. Kuiry, and S. Seal, Proc. R. Soc. Math. Phys. 460, 3569 (2004).
S. Deshpande, S. Patil, S.V.N.T. Kuchibhatla, and S. Seal, Appl. Phys. Lett. 87, 133113 (2005).
X.Y. Zhang, J.J. Wei, H.X. Yang, X.F. Liu, W. Liu, C. Zhang, and Y.Z. Yang, Eur. J. Inorg. Chem. 2013, 4443 (2013).
R.H. Gao, D.S. Zhang, P. Maitarad, L.Y. Shi, T. Rungrotmongkol, H.R. Li, J.P. Zhang, and W.G. Cao, J. Phys. Chem. C 117, 10502 (2013).
J.C. Zhang, J.X. Guo, W. Liu, S.P. Wang, A.R. Xie, X.F. Liu, J. Wang, and Y.Z. Yang, Eur J. Inorg. Chem. 6, 969 (2015).
W.P. Shan, F.D. Liu, H. He, X.Y. Shi, and C.B. Zhang, Chem. Commun. 47, 8046 (2011).
J.Y. Luo, M. Meng, J.S. Yao, X.G. Li, Y.Q. Zha, X. Wang, and T.Y. Zhang, Appl. Catal. B Environ. 87, 92 (2009).
G. Jacobs, L. Williams, U. Graham, G.A. Thomas, D.E. Sparks, and B.H. Davis, Appl. Catal. Gener. 252, 107 (2003).
X.F. Wan, D. Goberman, L.L. Shaw, G.S. Yi, and G.M. Chow, Appl. Phys. Lett. 96, 3371678 (2010).
A. Hornes, G. Munuera, A. Fuerte, M.J. Escudero, L. Daza, and A. Martinez-Arias, J. Power Sources 196, 4218 (2011).
A. Kaushik, P.R. Solanki, A.A. Ansari, S. Ahmad, and B.D. Malhotra, Nanotechnology 20, 055105 (2009).
A.A. Ansari, P.R. Solanki, and B.D. Malhotra, Appl. Phys. Lett. 92, 263901 (2008).
D.S. Zhang, Y.L. Qian, L.Y. Shi, H.L. Mai, R.H. Gao, J.P. Zhang, W.J. Yu, and W.G. Cao, Catal. Commun. 26, 164 (2012).
G. Jacobs, L. Williams, U. Graham, D. Sparks, and B.H. Davis, J. Phys. Chem. B 107, 10398 (2003).
N. Thovhogi, A. Diallo, A. Gurib-Fakim, and M. Maaza, J. Alloy. Compd. 647, 392 (2015).
S. Khamlich, B.D. Ngom, C.K. Kotsedi, K. Bouziane, E. Manikandan, and M. Maaza, Surf. Rev. Lett. 21, 1450001 (2014).
S. Mahammadunnisa, P.M.K. Reddy, N. Lingaiah, and C. Subrahmanyam, Catal. Sci. Technol. 3, 730 (2013).
Q. Fu, W.L. Deng, H. Saltsburg, and M. Flytzani-Stephanopoulos, Appl. Catal. B Environ. 56, 57 (2005).
S.X. Cai, D.S. Zhang, L. Zhang, L. Huang, H.R. Li, R.H. Gao, L.Y. Shi, and J.P. Zhang, Catal. Sci. Technol. 4, 93 (2014).
T.Y. Yu, J. Zeng, B. Lim, and Y.N. Xia, Adv. Mater. 22, 5188 (2010).
M. Balaguer, C. Solis, S. Roitsch, and J.M. Serra, Dalton T 43, 4305 (2014).
L. Liao, H.X. Mai, Q. Yuan, H.B. Lu, J.C. Li, C. Liu, C.H. Yan, Z.X. Shen, and T. Yu, J. Phys. Chem. C 112, 9061 (2008).
S. Banerjee, P.S. Devi, D. Topwal, S. Mandal, and K. Menon, Adv. Funct. Mater. 17, 2847 (2007).
C. Laberty-Robert, J.W. Long, K.A. Pettigrew, R.M. Stroud, and D.R. Rolison, Adv. Mater. 19, 1734 (2007).
H.J. Lang, K. Kunstler, and G. Tomandl, Solid State Ionics 157, 189 (2003).
Z.P. Qu, F.L. Yu, X.D. Zhang, Y. Wang, and J.S. Gao, Chem. Eng. J. 229, 522 (2013).
M. Li, Z.G. Liu, Y.H. Hu, Z.X. Shi, and H.Q. Li, Colloid Surface A 301, 153 (2007).
D. Channei, B. Inceesungvorn, N. Wetchakun, S. Ukritnukun, A. Nattestad, J. Chen, and S. Phanichphant, Sci. Rep. Uk 4, 5757 (2014).
V.S. Marques, L.S. Cavalcante, J.C. Sczancoski, A.F.P. Alcantara, M.O. Orlandi, E. Moraes, E. Longo, J.A. Varela, M.S. Li, and M.R.M.C. Santos, Cryst. Growth Des. 10, 4752 (2010).
A.A. Ansari, S.P. Singh, and B.D. Malhotra, J. Alloy. Compd. 509, 262 (2011).
A.A. Ansari, A. Kaushik, P.R. Solanki, and B.D. Malhotra, Electrochem. Commun. 10, 1246 (2008).
A.A. Ansari, P.R. Solanki, and B.D. Malhotra, Sensor Lett. 7, 64 (2009).
A.A. Ansari, S.P. Singh, N. Singh, and B.D. Malhotra, Spectrochim Acta A 86, 432 (2012).
A. Thurber, K.M. Reddy, V. Shutthanandan, M.H. Engelhard, C. Wang, J. Hays, and A. Punnoose, Phys. Rev. B 76, 165435 (2007).
T. Masui, K. Fujiwara, K. Machida, G. Adachi, T. Sakata, and H. Mori, Chem. Mater. 9, 2197 (1997).
B. Tatar, E.D. Sam, K. Kutlu, and M. Urgen, J. Mater. Sci. 43, 5102 (2008).
P. Patsalas, S. Logothetidis, L. Sygellou, and S. Kennou, Phys. Rev. B 68, 035104 (2003).
C.N. Xian, S.F. Wang, C.W. Sun, H. Li, S.W. Chan, and L.Q. Chen, Chin. J. Catal. 34, 305 (2013).
P. Maitarad, J. Han, D.S. Zhang, L.Y. Shi, S. Namuangruk, and T. Rungrotmongkol, J. Phys. Chem. C 118, 9612 (2014).
L. Meng, A.P. Jia, J.Q. Lu, L.F. Luo, W.X. Huang, and M.F. Luo, J. Phys. Chem. C 115, 19789 (2011).
B.M. Reddy, L. Katta, and G. Thrimurthulu, Chem. Mater. 22, 467 (2010).
H.Q. Zhu, Z.F. Qin, W.J. Shan, W.J. Shen, and J.G. Wang, J. Catal. 225, 267 (2004).
H.C. Yao and Y.F.Y. Yao, J. Catal. 86, 254 (1984).
Acknowledgement
The authors are grateful for financial support to King Saud University Deanship of Scientific Research, College of Sciences, Research Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, N., Ansari, A.A., Labis, J.P. et al. Impact of Ni Ion-Doping on Structural, Optoelectronic and Redox Properties of CeO2 Nanoparticles. J. Electron. Mater. 47, 2557–2564 (2018). https://doi.org/10.1007/s11664-018-6088-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-6088-x