Abstract
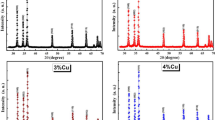
Fe-doped ZnO powders have been synthesized by the coprecipitation method using zinc nitrate [Zn(NO3)2·6H2O] as starting material, urea [CO(NH2)2] as precipitator, and ferric nitrate [Fe(NO3)3·9H2O] as doping source. The microstructure of the prepared powders has been characterized by x-ray diffraction and scanning electron microscopy. Results show that, when the molar ratio of Fe to (Zn + Fe) was less than 0.09, the prepared powder was ZnO(Fe) solid solution, and the ZnFe2O4 impurity phase appeared when the Fe doping content was further increased. The electric permittivity in the frequency range of 8.2 GHz to 12.4 GHz and the average infrared emissivity in the wavelength range of 8 μm to 14 μm have been determined for the prepared powders. The average infrared emissivity decreased with increasing Fe doping content. The real (ε′) and imaginary part (ε″) of the permittivity of the prepared powders showed opposite trends. When the molar ratio of Fe to (Zn + Fe) was 0.03, the prepared Fe-doped ZnO powder demonstrated the best microwave absorption in the frequency range of 8.2 GHz to 12.4 GHz.
Similar content being viewed by others
References
T.-H. Ting, K.-H. Wu, J.-S. Hsu, M.-H. Chuang, and C.-C. Yang, J. Chin. Chem. Soc. 55, 724 (2008).
Z.L. Wang, MRS Bull. 37, 814 (2012).
M.H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo, and P. Yang, Science 292, 1897 (2001).
Z.L. Wang, Mater. Sci. Eng. R 64, 33 (2009).
Y.-J. Chen, F. Zhang, G.-G. Zhao, X.-Y. Fang, H.-B. Jin, P. Gao, C.-L. Zhu, M.-S. Cao, and G. Xiao, J. Phys. Chem. C 114, 9239 (2010).
R.F. Zhuo, H.T. Feng, J.T. Chen, D. Yan, J.J. Feng, H.J. Li, B.S. Geng, S. Cheng, X.Y. Xu, and P.X. Yan, J. Phys. Chem. C 112, 11767 (2008).
Y.J. Chen, M.S. Cao, T.H. Wang, and Q. Wan, Appl. Phys. Lett. 84, 3367 (2004).
L. Kong, X.W. Yin, L.T. Zhang, and L.F. Cheng, J. Am. Ceram. Soc. 95, 3158 (2012).
T. Xin and K.-A. Hu, Mater. Sci. Eng. B 139, 119 (2007).
Y. Wang, F. Luo, L. Zhang, D.M. Zhu, and W.C. Zhou, Ceram. Int. 39, 8723 (2013).
Z.W. Zhou, L.S. Chu, and S.C. Hu, Mater. Sci. Eng. B 126, 93 (2006).
R.F. Zhuo, L. Qiao, H.T. Feng, J.T. Chen, D. Yan, Z.G. Wu, and P.X. Yan, J. Appl. Phys. 104, 094101 (2008).
H. Li, Y. Huang, G. Sun, X. Yan, Y. Yang, J. Wang, and Y. Zhang, J. Phys. Chem. C 114, 10088 (2010).
M.-S. Cao, X.-L. Shi, X.-Y. Fang, H.-B. Jin, Z.-L. Hou, W. Zhou, and Y.-J. Chen, Appl. Phys. Lett. 91, 203110 (2007).
Z.W. Zhou, S.K. Liu, and L.X. Gu, J. Appl. Polym. Sci. 80, 1520 (2001).
D.M. Zhu, K. Li, F. Luo, and W.C. Zhou, Appl. Surf. Sci. 255, 6145 (2009).
Y.-H. Yao and Q.-X. Cao, Chin. Phys. B 21, 124205 (2012).
S.Y. Zhang and Q.X. Cao, Adv. Mater. Res. 399–401, 880 (2012).
X.L. Su, W.C. Zhou, J. Xu, Z.M. Li, F. Luo, and D.M. Zhu, J. Alloys Compd. 492, L16 (2010).
S.A. Degterov, E. Jak, P.C. Hayes, and A.D. Pelton, Metall. Mater. Trans. B 32, 643 (2001).
P. Li, H. Liu, Y.-F. Zhang, Y. Wei, and X.-K. Wang, Mater. Chem. Phys. 106, 63 (2007).
Y.P. Chen, G.Y. Xu, T.C. Guo, and N. Zhou, Sci. China Technol. Sci. 55, 623 (2012).
V. Musat, B. Teixeira, E. Fortunato, R.C.C. Monteiro, and P. Vilarinho, Surf. Coat. Technol. 180–181, 659 (2004).
X.X. Yan and G.Y. Xu, J. Alloys Compd. 491, 649 (2010).
A.V. Moholkara, S.M. Pawara, K.Y. Rajpurea, V. Ganesanb, and C.H. Bhosalea, J. Alloys Compd. 464, 387 (2008).
X. Su, Y. Jia, J. Wang, J. Xu, X. He, C. Fu, and S. Liu, Ceram. Int. 39, 3651 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, X., Jia, Y., Liu, X. et al. Preparation, Infrared Emissivity, and Dielectric and Microwave Absorption Properties of Fe-Doped ZnO Powder. J. Electron. Mater. 43, 3942–3948 (2014). https://doi.org/10.1007/s11664-014-3218-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-014-3218-y