Abstract
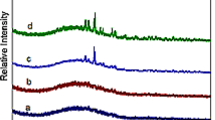
The structural roles of alkali and calcium cations are important for understanding the physical and chemical properties of aluminosilicate melts and glasses. Recently, oxygen-17 nuclear magnetic resonance (17O NMR) studies of calcium–sodium aluminosilicate glasses showed that these structural roles are not randomly given, but rather each cation has its own preferential role. However, the relationship between cation type and role preference in calcium aluminosilicate glass is not completely understood. In the present study, the structural roles of lithium, sodium, and potassium cations in selected calcium aluminosilicate glasses are investigated using 17O solid-state NMR experiments. Data from these experiments clearly show that potassium cations have a notably stronger tendency to act as charge compensators within the network structure, compared to sodium and lithium cations. The result of 17O NMR experiment also showed that sodium and lithium cations in part act as network modifier alongside with calcium cations.



Similar content being viewed by others
References
H. Y. Chang, T. F. Lee and T. Ejima: Trans. Iron Steel Inst. Jpn., 1987, vol. 27, pp. 797-804.
S. Sukenaga, N. Saito, K. Kawakami and K. Nakashima: ISIJ Int., 2006, vol. 46, pp. 352-58.
W. H. Kim, I. Sohn and D. J. Min: Steel Res. Int., 2010, vol. 81, pp. 735-41.
I. Sohn and D. J. Min: Steel Res. Int., 2012, vol. 83, pp. 611-30.
G. H. Zhang and K. C. Chou: Metal. Mater. Trans. B, 2012, vol. 43, pp. 841-48.
T. Higo, S. Sukenaga, K. Kanehashi, H. Shibata, T. Osugi, N. Saito and K. Nakashima: ISIJ Int., 2014, vol. 54, pp. 2039-44.
L. Cormier, D. R. Neuville and G. Calas: J. Am. Ceram. Soc., 2005, vol. 88, pp. 2292-99.
M. J. Toplis and D. B. Dingwell: Geochim. Cosmochim. Acta, 2004, vol. 68, pp. 5169-88.
G. Urbain, Y. Bottinga and P. Richet: Geochim. Cosmochim. Acta, 1982, vol. 46, pp. 1061-72.
K. J. D. MacKenzie and M. E. Smith: Multinuclear solid-state NMR of inorganic materials. 1st ed. (Pergamon, Oxford, 2002).
S. K. Lee and S. Sung: Chem. Geol., 2008, vol. 256, pp. 326-33.
A. Pedone, E. Gambuzzi and M. C. Menziani: J. Phys. Chem. C, 2012, vol. 116, pp. 14599-609.
B. O. Mysen and P. Richet: Silicate Glasses and Melts: Properties and Structure. 1st ed. (Elsevier, Amsterdam, 2005).
S. Sukenaga, T. Nagahisa, K. Kanehashi, N. Saito and K. Nakashima: ISIJ Int., 2011, vol. 51, pp. 333-35.
A.E. Geissberger and P.J. Bray: J. Non-Cryst. Solids, 1983, vol. 54, pp. 121-37.
L. Frydman and J.S. Harwood: J. Am. Chem. Soc., 1995, vol. 117, pp. 5367-68.
J.P. Amoureux, C. Fernandez, and S. Steuernagel: J. Magn. Reson., Ser A, 1996, vol. 123, pp. 116–18.
J. P. Amoureux, C. Huguenard, F. Engelke and F. Taulelle: Chem. Phys. Lett., 2002, vol. 356, pp. 497-504.
K. Kanehashi, K. Shimoda and K. Saito: Tetsu-to-Hagané, 2009, vol. 95, pp. 321-30.
J. F. Stebbins and Z. Xu: Nature, 1997, vol. 390, pp. 60-62.
J. F. Stebbins, J. V. Oglesby and S. K. Lee: Chem. Geol., 2001, vol. 174, pp. 63-75.
J.F. Stebbins, E.V. Dubinsky, K. Kanehashi and K.E. Kelsey: Geochim. Cosmochim. Acta, 2008, vol. 72, pp. 910-25.
S. K. Lee and J. F. Stebbins: J. Phys. Chem. B, 2003, vol. 107, pp. 3141-48.
H. Maekawa, P. Florian, D. Massiot, H. Kiyono and M. Nakamura: J. Phys. Chem., 1996, vol. 100, pp. 5525-32.
P. Florian, K. E. Vermillion, P. J. Grandinetti, I. Farnan and J. F. Stebbins: J. Am. Chem. Soc., 1996, vol. 118, pp. 3493-97.
S.K. Lee and J.F. Stebbins: J. Non-Cryst. Solids, 2000, vol. 270, pp. 260-64.
J. F. Stebbins, J. S. Wu and L. M. Thompson: Chem. Geol., 2013, vol. 346, pp. 34-46.
K. Shimoda, Y. Tobu, K. Kanehashi, K. Saito and T. Nemoto: Solid State Nucl. Magn. Reson., 2006, vol. 30, pp. 198-202.
H. Uhlig, M. J. Hoffmann, H. P. Lamparter, F. Aldinger, R. Bellissent and S. Steeb: J. Am. Ceram. Soc., 1996, vol. 79, pp. 2833-38.
X. Y. Xue and J. F. Stebbins: Phys. Chem. Miner., 1993, vol. 20, pp. 297-307.
W. E. Jackson, G. E. Brown and C. W. Ponader: J. Non-Cryst. Solids, 1987, vol. 93, pp. 311-22.
R. D. Shannon: Acta Crystallogr. Sect. A, 1976, vol. 32, pp. 751-67.
D. R. Neuville, L. Cormier, V. Montouillout, P. Florian, F. Millot, J. C. Rifflet and D. Massiot: Am. Mineral., 2008, vol. 93, pp. 1721-31.
K. E. Kelsey, J. R. Allwardt and J. F. Stebbins: J. Non-Cryst. Solids, 2008, vol. 354, pp. 4644-53.
X. Y. Xue: Solid State Nucl. Magn. Reson., 2010, vol. 38, pp. 62-73.
Acknowledgments
The authors (H.S., K.N., N.S., and S.S.) are grateful for the financial support from the Cooperative Research Program of “Network Joint Research Center for Materials and Devices”, IMRAM Tohoku University. This work was financially supported in part by a Grant-in-Aid for Scientific Research (C) Grant (No. 25420792) from the Japan Society for the Promotion of Science (JSPS) and research fund by IMRAM, Tohoku University. The authors would like to acknowledge and thank Dr. Takafumi Takahashi and Mr. Tatsuya Nishiura (Nippon Steel & Sumitomo Metal Corporation) for technical support in NMR measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 18, 2015.
Rights and permissions
About this article
Cite this article
Sukenaga, S., Kanehashi, K., Shibata, H. et al. Structural Role of Alkali Cations in Calcium Aluminosilicate Glasses as Examined Using Oxygen-17 Solid-State Nuclear Magnetic Resonance Spectroscopy. Metall Mater Trans B 47, 2177–2181 (2016). https://doi.org/10.1007/s11663-016-0689-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0689-7