Abstract
The slab reheating process of binary iron-aluminum alloys and an industrial TRIP steel grade has been investigated in both dry and wet atmospheres. The presence of water vapor has a significant effect on the overall scale growth and internal corrosion depth. Heating rate greatly influences the porosity of the surface oxide layer with the surface getting more porous at faster heating rates. Nitride formation could be suppressed in the presence of water vapor, leading to a reduction of internal corrosion depth and a better formability of the final material. Experimental results were compared to thermodynamic predictions and critically discussed.
Similar content being viewed by others
Introduction
Water vapor has an undoubtedly dramatic effect on the oxidation behavior of metals at elevated temperatures.[1–3] Especially in complex atmospheres like in a slab reheat furnace, a fundamental understanding of the corrosion behavior of materials like advanced high-strength steels or low-density steels with a high aluminum content[4] represents a significant technical advantage. Besides the many works on the effect of humidity,[5,6] a clear technical benefit for steel manufacture is still missing. However, it is often readily possible to remove an external iron oxide scale layer in silicon-free steels, formed during casting, reheating, and hot rolling.[7] If the internal oxidation underneath the oxide layer is too heavy,[8,9] this can cause problems during downstream processing (e.g., crevice corrosion in pickling solutions[10] or uncoated spots during galvanizing). By designing the gas composition in a reheat furnace in such a way that an outer scale layer may form, the internal oxide formation could be reduced.[2] However, great care needs to be taken, since oxygen (and iron) transport through this scale layer will be increased in the presence of water vapor.[11,12] Hence it is the aim of this work to characterize the oxidation behavior in dry and wet atmospheres to help inventing optimized strategies for steel production with a minimal formation of internal oxides. For high-Al AHSS grades, internal nitridation should also be considered.[4,6]
Experimental
Materials
Two binary iron-aluminum model alloys with an aluminum content of 1 and 5 wt pct Al and one industrial TRIP steel composition (all compositions listed in Table I) were cut to 15 mm × 15 mm × 2 mm pieces and then ground on silicon carbide grinding papers through 1200 grit. After grinding, the surfaces were cleaned using deionized water and ethanol to remove any residual oils or other impurities before heat treatment.
Experimental Parameters
Two different gas mixtures were used to study the corrosion behavior. One was an N2, 10 vol pct CO2, 8 vol pct H2O, 2 vol pct O2 (+40 °C dew point) mixture to mimic the behavior in an industrial slab reheat furnace. Another mixture was dry synthetic nitrogen, containing 6 ppm oxygen to unravel the effects of water vapor and carbon dioxide on the corrosion process. Although the exact water vapor content in the dry gas mixture could not be measured, we assumed an upper limit of 1 ppm H2O, as mentioned on the gas cylinder. Water vapor content in the wet atmosphere was controlled by bubbling the dry gas through a water bath, maintained at a temperature corresponding to the desired dew point.[13] Experiments were conducted in each atmosphere separately, corresponding to oxygen fugacities at 1558 K (1285 °C) of ≈0.02 atm for the reheat furnace atmosphere and 6×10−6 atm in the dry gas. Both oxygen fugacities can lead to the formation of a thermodynamically stable scale layer containing iron oxides.[14]
The temperature profile was designed to replicate slab reheating conditions.[15] Samples were heated in an alumina tube furnace from room temperature to 1558 K (1285 °C) with three different heating rates (12.6, 126, and 1260 K min−1) and then held isothermally for up to 200 minutes. The fastest heating rate could be realized by pushing the sample from the cold zone directly into the hot furnace, using the experimental set-up, illustrated in Figure 1(a). An overview of experimental conditions is briefly given in Table II.
After heat treatment, the samples were cooled in the flowing gas mixture. Both the temperature program during the experiments and the experimental set-up are shown in Figure 1. Cross sections were obtained by mounting the samples in epoxy and then sectioned using a low-speed saw. The samples were then ground using successively finer SiC papers and then polished to a 1 µm finish using a series of diamond suspensions. Micrographs were generated using scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM–EDS, Quanta 600, FEI, Hillsboro, OR).
Results and Discussion
Very stable conditions for temperature and humidity could be achieved throughout the exposure. A minor amount of overheating was observed at the beginning of some experiments, which occurred as a consequence of the fast temperature rise in the set-temperature program. In order to give the reader a better impression on the influence of individual process parameters—gas atmosphere, heating rate, and soaking time—these effects are discussed individually.
Effect of Gas Atmosphere
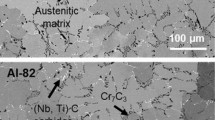
In dry nitrogen/oxygen, a thin oxide layer measuring up to 5 µm was observed, which is similar to previous studies.[4] Cross sections of TRIP steel, oxidized in dry nitrogen/oxygen, can be seen in Section III–B (Figure 5(a)). Severe corrosive attack only occurred in the reheat furnace atmosphere,[16] showing massive formation of iron oxides at the surface. In all alloys, three different zones were identified, as shown in Figure 2. The outermost zone occurred due to external scale formation and consisted predominately of Fe3O4. The intermediate zone includes the original sample surface (dotted line in Figure 2) and was composed of mixed iron/alloy element oxides—Fe3O4 and FeAl2O4—with manganese being distributed between both oxide phases for the TRIP steel. The internal oxidation zone, labeled “alloy,” contained selective oxidation products of the alloy additions such as Al2O3 and AlN. Their size ranged between 2 and 20 µm. No significant amounts of manganese could be identified in these precipitates. Little to no differences in the external scale formation were observed between TRIP steel and binary iron-1 pct aluminum.
Whereas the formation of pure iron oxides is thermodynamically expected in dry nitrogen/oxygen as well as in reheat furnace gas,[14] the huge differences in the oxidation behavior suggests that either the presence of water vapor, which is known to cause massive corrosion,[17,18] or carbon dioxide[16,19] has a significant impact on corrosion kinetics.[3,20] Furthermore, oxygen transport from the atmosphere into the sample has a highly transient character. Due to the formation of an outer iron oxide layer, oxygen transport will be limited by the penetration of oxygen atoms through the oxide layer[21] as well as by the decomposition of wustite,[22] which effectively reduces the internal corrosion depth (i.e., selective oxidation of alloy elements underneath the iron oxide zone).
Effect of Heating Rate
In the reheat furnace atmosphere, no significant changes of the surface oxide morphology could be observed for different heating rates. However in dry nitrogen/oxygen, the composition of the surface in binary iron-aluminum[4] clearly changes with faster heating rates. In the same atmosphere, the porosity of the oxide layer in TRIP steel increases with faster heating rates, as shown in Figure 3.
If the porous structure is caused by the evaporation of dissolved aluminum, aluminum-(oxy)hydroxide species[23] or is simply a consequence of poor adhesion of surface oxides, could not be clearly resolved. However, thermodynamic calculations of the corresponding partial pressures for volatile aluminum species (see Figure 4) show that trends are at least similar to what has been reported for Cr-oxide forming alloys with respect to the partial pressures of volatile Cr species in typical water vapor environments.[24] It is well known that CrO2(OH)2 evaporation will affect the thickness of the formed surface oxide layers and the corrosion behavior of the alloy.[17,20] Similarly, it is suggested that the formation of volatile aluminum (oxy-)hydroxides could at least play a minor role in pore formation, albeit to a much lesser extent. The proposed mechanisms from literature, leading to volatilization of aluminum oxide[23], are shown in Eqs. [1] through [4]:
Calculated partial pressures for pure aluminum and volatile aluminum (oxy-)hydroxides in reheating furnace atmospheres (a), dry atmosphere (b), and for selective oxidation studies[6] (c). The corresponding gas compositions are (a) N2, 10 vol pct CO2, 8 vol pct H2O, 2 vol pct O2; (b) N2, 6 ppm O2, 1 ppm H2O; and (c) N2, 2.5 vol pct H2, 1.08 vol pct H2O. Thermodynamic values of the volatile (oxy-)hydroxide species were taken from Ref. [23]
Partial pressures of volatile (oxy-)hydroxide species at high temperatures are all in the same order of magnitude, but significant differences do exist at lower temperatures. This is particularly important during the non-isothermal part of the heat treatment and shows a nice qualitative agreement with the change of the surface porosity at different heating rates. Furthermore, findings in pure Fe-Al alloys also report a higher Al concentration close to the surface during faster heating[4] which may help accelerate the formation of volatile species.
Effect of Soaking Time
Whereas the thickness of the outer iron oxide layer remains almost unchanged, the internal oxidation depth in all alloys significantly increased for longer dwell times. Here, it is important to mention that the morphology of the formed aluminum precipitates does not seem to change during thermal exposure. As can be seen in the cross sections shown in Figure 5, the zone of fine-grained aluminum precipitates in the zero-dwell experiment has the same appearance as those in the near-surface area of the sample with additional 200-minute exposure at 1558 K (1285 °C). The formation of coarser precipitates could only be observed toward the corrosion front, where the molar fluxes of aluminum and nitrogen/oxygen would be expected to become equal for longer exposure times. In agreement with predictions from thermodynamic simulations of local phase formation in binary iron-aluminum,[6] EDX images in Figure 5 show that the area close to the sample surface (top of Figure 5(a)) consists of the stable oxides. The thermodynamically less favorable nitride phases can then be found at lower oxygen activities close to the corrosion front—since nitrogen also diffuses faster than oxygen.[9] This can also be seen from the EDX measurements in Figure 5, which were taken at the interface between aluminum nitrides and aluminum oxides. Here, the upper part of the precipitates (closer to the sample surface) consists of oxides, whereas the bottom part (closer to the internal corrosion front) does not show any oxide, which indicates nitride formation. During thermal exposure, the formed aluminum nitrides turn into oxides as soon as the local oxygen activity in the material reaches a critical value. Hence nitrogen will be released into the metal lattice, where it diffuses toward the corrosion front prior to combining with the metallic alloy addition. Macroscopically, this effect looks like oxygen is “pushing” the nitrogen further inwards,[6,9] although it is in fact a transformation from the initially formed nitrides into the thermodynamically more stable oxide phases. This result is particularly interesting for further processing steps, as it suggests that the morphology of the initially formed aluminum precipitates is determined by nitrides, which may later be transformed into oxides.
SEM cross sections of TRIP steel, oxidized in N2, 6 ppm O2 for different dwell times (top, heating rate: 12.6 K min−1) and EDX images of the cross section of Fe-5Al, kept for 200 min at 1558 K (1285 °C, bottom). The dotted line in (a) represents the corrosion depth of the no-dwell experiment and the EDX images in (b) were taken at the interface between internal oxides and nitrides (Color figure online)
Little to no signs of nitride formation could be detected for experiments in the reheat furnace atmosphere. The high oxygen fugacity in this atmosphere leads to massive formation of oxides at the surface, which will effectively block nitrogen uptake from the gas mixture. Hence the only nitrides that form originate from the nitrogen content in the material before heat treatment (see Table I).
Effect of Manganese
No clear signs of internal precipitates along the grain boundaries could be observed in the presence of manganese (TRIP steel), as shown in Figure 6. Remarkably, the depth of the internal corrosion zone in TRIP steel is bigger than that in pure iron-aluminum, but both values do not exceed the predictions from numerical simulations.[6,8] This suggests that manganese slightly accelerates internal oxidation in reheat furnace conditions but forms round-shaped precipitates instead of grain boundary oxides. Although selective oxidation of pure ternary Fe-2Mn-1Al samples[8] exhibits a clear tendency toward grain boundary oxide formation, it needs to be pointed out that this discrepancy is likely to be caused by the different oxygen fugacities in these atmospheres and the presence/absence of an outer iron oxide layer that affects oxidation kinetics.
SEM cross sections of the internal corrosion zone in manganese-free Fe-1Al (a), and TRIP steel (1.45 wt pct Al and high Mn, (b)), oxidized in N2, 10 vol pct CO2, 8 vol pct H2O, 2 vol pct O2 (+40 °C dew point). The heating rate in both experiments was 12.6 K min−1, followed by isothermal dwelling at 1558 K (1285 °C) for 200 min
The formation of internal (and grain boundary) oxide/nitride precipitates is particularly important for subsequent forming processes. It is quite easy to remove an outer scale layer, but the formation of brittle oxides along grain boundaries and precipitates inside the metal is usually connected with expensive pickling treatments, aiming to remove the corrosion-affected zone and leading to a significant loss of material. Moreover, it needs to be mentioned that mechanical properties change in the presence of inclusions.[25]
It needs to be pointed out that the TRIP steel composition, reported here, appears to be incomplete in the sense that it also contains a few minor alloy elements. Although these additions were not mentioned for reasons of confidentiality, the obtained results were in good agreement with the corrosion behavior of binary alloys, modeling predictions, and other studies on pure alloy systems. Thus, it can be concluded that minor element additions play only a minor role (if any) for the scope of this work.
Conclusion
High-temperature corrosion experiments of binary iron-aluminum alloys and an industrial TRIP steel grade have been performed. Temperature and gas composition have been adapted to mimic typical slab reheat furnace conditions [1558 K, (1285 °C), N2/CO2/O2/H2O atmosphere]. Corrosion in dry nitrogen/oxygen was investigated to unravel the effects of individual gas components and oxygen fugacity in the gas on the corrosion behavior of the alloy.
It could be found that the length of the isothermal heat exposure significantly influences the total corrosion depth, whereas faster heating leads to a more porous (or tree-like) structure of the formed surface oxides. Furthermore, the presence of an oxide layer at the surface (high-oxygen fugacity gases) will block nitrogen uptake which reduces the presence of nitrides and the depth of the internal corrosion zone. Nitride formation—if present—will define the morphology of the formed aluminum precipitates, which may later transform into the thermodynamically stable oxide phases. This finding is in good agreement with results from thermodynamic simulations of the local phase formation. Hence, nitride formation can affect the mechanical properties of the material (e.g., dispersion-hardened steels) prior to the rolling process.
No clear signs of grain boundary oxidation could be observed in the TRIP steel. Most likely, the transient conditions in the experiment in combination with the presence of manganese in the alloy lead to the reduction of grain boundary oxide formation that may otherwise weaken the cohesion between individual grains. This results in a better formability of the TRIP material during the subsequent hot rolling steps, since the outer scale layer is relatively easy to be removed but the internal precipitation zone is not.
References
E. Essuman, G.H. Meier, J. Zurek, M. Hänsel, L. Singheiser, W.J. Quadakkers: Scripta Mater., 57(2007), 845.
H.J. Grabke, M. Schütze: Oxidation of Intermetallics: Wiley VCH, Weinheim (2008)
D.J. Young: High Temperature Oxidation and Corrosion in Metals, Elsevier, Oxford (2008).
J. Bott, H. Yin, S. Sridhar: Met. Mater. Trans B, 45(2014), 2222.
M. Fukumoto, S. Maeda, S. Hayashi, T. Narita: Oxid. Met., 55(2001), 401.
J.H. Bott, H. Yin, S. Seetharaman, M. Auinger: Corros. Sci., 91(2015), 37.
H.K.D.H. Bhadeshia, R.W.K. Honeycombe: Steels—Microstructure and Properties, Butterworth-Heinemann, Cambridge (2006).
M. Auinger, V.G. Praig, B. Linder, H. Danninger: Corros. Sci., 96(2015), 133.
M. Auinger. E.M. Müller-Lorentz, M. Rohwerder: Corros. Sci., 90(2015), 503.
A.J. Bard, M. Stratmann: Encyclpopedia of Electrochemistry, Wiley-VCH, Weinheim (2002).
W.J. Quadakkers, J. Zurek, M. Hänsel: JOM, 61(2009) 44.
D.J. Young, H. Yin. Oxid. Met., 79(2013) 445.
M. Auinger, D. Vogel, A. Vogel, M. Spiegel, M. Rohwerder: Rev. Sci. Instr., 84(2013), 085108.
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R. Ben Mahfoud, J. Melancon, A.D. Pelton, S. Petersen: Calphad, 26(2002) 189.
J.H. Bott. Subsurface Aluminum Nitride Formation in Iron-Aluminum Allloys. Ph.D. Thesis, Carnegie Mellon University, Pittsburgh, 2014.
R. Bredesen, P. Kofstad: Oxid. Met., 34(1990), 361.
E. Essuman, G.H. Meier, J. Zurek, M. Hänsel, W.J. Quadakkers: Oxid. Met., 69(2009), 143.
H. Yin, S.L.I. Chan, W.Y.D. Yuen, D.J. Young: Oxid. Met., 77(2012), 305.
W.J. Quadakkers, T. Olszewski, J. Piron-Abellan, V. Shemet, L. Singheiser: Mat. Sci. Forum, 696(2011), 194.
N. Birks, G.H. Meier, F.S. Pettit: Introduction to High Temperature Oxidation of Metals, Cambridge University Press, Cambridge (2006).
A. Holt, P. Kofstad: Solid State Ionics, 69(1994), 127.
M.H. Davies, M.T. Simnad, C.E. Birchenall: T. Metall. Soc. AIME, 10(1951), 889.
N. Jacobson, D. Myers, E. Opila, E. Copland: J. Phys. Chem. Solids, 66(2005), 471.
H. Asteman, J.E Svensson, L.G. Johansson: Corros. Sci., 44(2002), 2635.
P.C. van Wiggen, H.C.F. Rozendaal, E.J. Mittemeijer: J. Mat. Sci., 20(1985), 4561.
Acknowledgments
Financial support from ArcelorMittal Global R&D (East Chicago, IN, United States) and the Deutsche Forschungsgemeinschaft (Germany, DFG Project No. 598257) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 28, 2015.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bott, J.H., Yin, H., Sridhar, S. et al. Influence of Gas Composition and Exposure Cycle on the Formation of Surface and Subsurface Oxides in Iron-Aluminum-Based Alloys at High Temperatures. Metall Mater Trans B 47, 2157–2163 (2016). https://doi.org/10.1007/s11663-016-0671-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0671-4