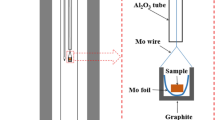
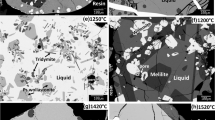
Abstract
The solubility of vanadium oxide in the Al2O3-CaO(30 mass pct)-SiO2 system and Al2O3-CaO(35 mass pct)-SiO2 system was determined experimentally at 1873 K (1600 °C) and at a fixed oxygen potential of 9.37 × 10−11 bar. EPMA microanalyses were employed to identify the phases and their compositions in the quenched samples. The solubility of vanadium oxide in the liquid phase was found to decrease with increasing CaO content in the liquid. The vanadium oxide solubility was especially low when both CaO and Al2O3 contents were high in the liquid phase. The maximum solubility of vanadium oxide was up to 7 mass pct (as V). Two solid phases were found, a solid solution of Al2O3 and vanadium oxide and an Al2O3-rich solid phase with 16.7 mass pct V2O3. The Al2O3 solubility in the solid solution was found to increase with increasing Al2O3 content in the liquid, the impact of the CaO content in the liquid on the solubility of Al2O3 in V2O3 was found to be small. The Al2O3-rich solid phase was identified as the mineral hibonite with fractionation of V into the crystal structure.









Similar content being viewed by others
References
M. Lindvall, J. Gran, and D. Sichen: CALPHAD 2014, vol. 47, pp. 50–55.
M. Lindvall, E. Rutqvist, G. Ye, J. Bjorkvall, and D. Sichen, Steel Res. Int., 2010, vol. 81, pp. 105–11.
E.F. Osborn and A. Muan: Phase Equilibrium Diagrams of Oxide Systems, American Ceramic Society, Ohio, 1960, Plate 1.
R. Burns and V. Burns: J. Geophys. Res., 1984, vol. 89, pp. C313–21.
J. Armstrong, G. Meeker, J. Huneke, and G. Wasserburg: Geochim. Cosmochim. Acta, 1982, vol. 46, pp. 575–95.
G. Macpherson, M.M. Bar-Matthews, T. Tanaka, E. Olsen, and L. Grossman: Geochim. Cosmochim. Acta, 1983, vol. 47, pp. 823–39.
A. Goresy and P. Ramdohr: Meteoritics, 1980, vol. 15, p. 286.
M. Harder and H. Mueller-Buschbaum: Z. Naturforsch. B Anorg. Chem. Org. Chem., 1977, vol. 32, pp. 833–34.
Acknowledgments
Funding and support from MISTRA, SSAB, LKAB, Ruukki, SSAB Merox, and Jernkontoret are gratefully acknowledged with special thanks to Dr. G. Ye and Dr. J. Björkvall of Swerea MEFOS. Thanks also to Stefan Andersson for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 16, 2014.
Rights and permissions
About this article
Cite this article
Lindvall, M., Sichen, D. Determination of Vanadium Solubility in the Al2O3-CaO(30 Mass Pct)-SiO2 and Al2O3-CaO(35 Mass Pct)-SiO2 System. Metall Mater Trans B 46, 733–740 (2015). https://doi.org/10.1007/s11663-014-0237-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0237-2