Abstract
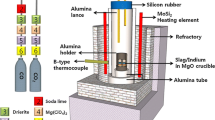
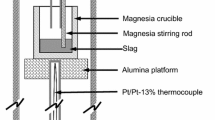
To understand the behavior of rhodium during its recovery process, the dissolution behaviors of rhodium in Na2O-SiO2 and in CaO-SiO2 slags at temperatures ranging from 1423 K to 1623 K (from 1150 °C to 1350 °C) and from 1773 K to 1873 K (from 1500 °C to 1600 °C), respectively, in an oxidizing atmosphere were investigated. The solubility of rhodium in the slags was found to increase with increasing oxygen partial pressure, temperature, and the basic oxide content. The correlation between the solubility of rhodium and the oxygen partial pressure suggested that rhodium dissolved into the slags as RhO1.5. The dissolution of rhodium was slightly endothermic: the enthalpy change of the dissolution of solid rhodium was determined to be 50 ± 10 kJ/mol for the 50(mass pct)Na2O-50SiO2; and 188 ± 94 kJ/mol for the 56(mass pct)CaO-44SiO2 slag systems. The increase in the solubility of rhodium with the basic oxide content indicated that rhodium exhibits acidic behavior in slags. The correlation between the solubility of rhodium and the sulfide capacity of the slags suggested that the ionic species of rhodium in slags is the rhodate ion, RhO −2 . The rhodate capacity of the slags was defined, and its application to estimate the possible rhodium content in various slag systems was proposed.










Similar content being viewed by others
References
J. Butler: Platinum 2012, Johnson Matthey Plc., Hertfordshire, 2012.
M. Benson, C.R. Bennett, J.E. Harry, M.K. Patel, and M. Cross: Resour., Conserv. Recycl., 2000, vol. 31, pp. 1–7.
C. Hagelüken: World Metall.–Erzmet. 2006, vol. 59, pp. 152–61.
T.H. Okabe, H. Nakada, and K. Morita: J. Surf. Sci. Soc. Jpn., 2008, vol. 29, no. 10, pp. 592–600.
S. Nakamura and N. Sano: Metall. Mater. Trans. B, 1997, vol. 28B, pp. 103–08.
S. Nakamura, K. Morita, and N. Sano: Metall. Mater. Trans. B, 1998, vol. 29B, pp. 411–14.
D.R. Swinbourne, S. Yan, and S. Salim: Trans. Inst. Min. Metall., Sect. C, 2005, vol. 114, pp. C23–C29.
H. Shuto, T.H. Okabe, and K. Morita: Mater. Trans., 2011, vol. 52, no. 10, pp. 1899–1904.
A. Paul and J.M. Parker: Phys. Chem. Glasses, 1975, vol. 16, no. 5, pp. 103–07.
P. Dable, M. Allibert, and J.C. Poignet: J. Am. Ceram. Soc., 2001, vol. 84, no. 5, pp. 1097–07.
I. Barin: Thermochemical Data of Pure Substances, VCH Verlagsgesellschaft, mbH, Weinheim, 1989.
F.D. Richardson and C.J.B. Fincham: J. Iron Steel Inst., London, 1954, pp. 4–15.
D.J. Sosinsky and I.D. Sommerville: Metall. Trans. B, 1986, vol. 17B, pp. 331–37.
J.A. Duffy and M. Ingram: J. Non-Cryst. Solids, 1976, vol. 21, pp. 373–410.
J.A. Duffy: Geochim. Cosmochim. Acta, 1993, vol. 57, pp. 3961–3970.
R. Inoue and H. Suito: Trans. Iron Steel Inst. Jpn., 1982, vol. 22, pp. 514–23.
A. Mendiboure, H. Eickenbusch, R. Schollhorn, and G.V. Subba Rao: J. Solid State Chem., 1987, vol. 71, pp. 19–28.
K.T. Jacob and Y. Waseda: J. Solid State Chem., 2000, vol. 150, pp. 213–220.
N.I. Zakharchenko: Russ. J. Appl. Chem., 2002, vol. 75, pp. 1629–31.
Acknowledgments
This research was supported the New Energy and Industrial Technology Development Organization (NEDO), Japan. The authors would like to express our thanks to Mr. Yuzuru Nakamura, Dowa Metals & Mining Co., Ltd., Japan, and Assoc. Prof. Dr. Takeshi Yoshikawa, Institute of Industrial Science, The University of Tokyo, Japan, for the fruitful advice and discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted February 27, 2012.
Rights and permissions
About this article
Cite this article
Wiraseranee, C., Okabe, T.H. & Morita, K. Dissolution Behavior of Rhodium in the Na2O-SiO2 and CaO-SiO2 Slags. Metall Mater Trans B 44, 584–592 (2013). https://doi.org/10.1007/s11663-013-9816-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-013-9816-x