Abstract
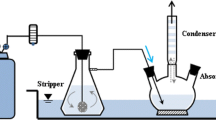
The communication presents an extension of the leaching process of the salt cake earlier developed by the present authors. The process describes the investigations in capturing the ammonia gas evolved by hydrolysis of AlN during aqueous leaching at 373 K (100 °C) by CO2-saturated water. The product, i.e., ammonium bicarbonate which is free of chlorides, is a value-added product and can find application in the fertilizer industry. The present method has the added advantage of fixing CO2 as well.




Similar content being viewed by others
References
“European Waste Catalogue and Hazardous Waste List”, Environmental Protection Agency, Ireland, Valid from 1 January 2002.
J.Y. Hwang, X. Huang, and Z. Xu: JMMCE, 2006, vol. 5, no. 1, pp. 47–62.
A. Gil and S.A. Korili: Environ Manage, ed. S.K. Sarkar, Intech Pub., 2010, pp. 149–58.
P. Li, M. Guo, M. Zhang, L.D. Teng, S. Seetharaman: Metall. Mater. Trans. B., 2012, vol. 43B, pp. 1220–30.
P. Li, M. Guo, M. Zhang, L.D. Teng, S. Seetharaman: Trans. IMM C., 2012, vol.121, no.3, pp.140–46.
P. Li, M. Zhang, L.D. Teng, and S. Seetharaman: Metall. Mater. Trans. B., 2012. doi:10.1007/s11663-012-9763-y.
Duncan, Proceedings of 20th Annual International Pittsburgh Coal Conference, Pittsburgh, 2003.
K.P. Resnik, J.T. Yeh, and H.W. Pennline: Int. J. Environ. Technol. Manag., 2004, vol. 4, nos. 1/2, pp. 89–104.
A.C. Yeh and H. Bai: Sci Total Environ., 1999, vol. 228, pp. 121–33.
F. Mani, M. Peruzzini, and P. Stoppioni: Green Chem., 2006, vol. 8, pp. 995–1000.
H. Bai and A. C. Yeh: Ind. Eng Chem. Res., 1997, vol. 36, pp. 2490–93.
A. Kocjan, A. Dakskobler, K. Krnel, and T. Kosmac: Cryst. Growth Des., 2012, vol. 12, no. 3, pp. 1299–1307.
K. Thomsen and P. Rasmussen: Chem. Eng. Sci., 1999, vol. 54, pp. 1787–1802.
R. Brooks: British Patent 742,386, 1953.
C.C. Shale, D.G. Simpson, and P.S. Lewis: Chem. Eng. Prog. Symp. Ser., 1971, vol. 67, p. 52.
J.E. Pelkie, P.J. Concannon, D.B. Manley, and B.E. Poling: Ind. Eng. Chem. Res., 1992, vol. 31, p. 2209.
P. Koubsky and V. Hladky: Int. Chem. Eng., 1976, vol. 16, p. 392.
M. Grayson and D. Eckroth Kirk-Othmer: Encyclopedia of Chemical Technology, 3rd ed., vol. 2. Wiley, New York, 1978.
E.C. Wagner: Ind. Eng. Chem., Anal. Ed., 1940, vol. 12, p. 711.
E.W. Meeker and E.C. Wagner: Ind. Eng. Chem., Anal. Ed., 1933, vol. 5, p. 396.
S. Fukumoto, T. Hookabe and T. Tsubakino: J. Mater. Sci., 2000, vol. 35, pp. 2743–48.
A. Kocjan, A. Dakskobler, K. Krnel, and T. Kosmac: J. Eur. Ceram. Soc., 2011, vol. 31, pp. 815–23.
J.W. Li, M. Nakamura, T. Shirai, K. Matsumaru, C. Ishizaki, and K. Ishizaki: J. Am. Ceram. Soc., 2006, vol. 89, no.3, pp. 937–43.
O. Levenspiel: Chemical Reaction Engineering, 2nd ed. Wiley, New York, 1972.
F. Montes, C.A. Rotz, and H. Chaoui: Trans. ASABE., 2009, vol. 52, no. 5, pp. 1707–19.
J. Ni: J. Agric. Eng. Res., 1999, vol. 72, no. 1, pp. 1–17.
The authors are thankful to the Iron and Steel Institute Research Institute Beijing, China, for their help with the chemical analyses. The authors are thankful to Stena Aluminum AB for the supply of salt cake samples. Partial financial support from the University of Science and Technology Beijing, China, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 21, 2012.
Rights and permissions
About this article
Cite this article
Li, P., Zhang, M., Teng, L. et al. Recycling of Aluminum Salt Cake: Utilization of Evolved Ammonia. Metall Mater Trans B 44, 16–19 (2013). https://doi.org/10.1007/s11663-012-9779-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9779-3