Abstract
The oxidation behaviors of composites SiAlON/MgAlON phases (β-SiAlON, 15R-SiAlON and MgAlON) synthesized from the residue during the leaching treatment of salt cake and corresponding synthetic samples were investigated in air by thermogravimetric measurements. Combined kinetics, viz. linear law + arctan law + parabolic law, are used to describe the kinetics of oxidation in isothermal mode. The oxidation studies reveal the effects of impurities, namely, Fe2O3 and CaO, present in the salt cake residue. The addition of Fe2O3 results in a lower activation energy and more aggressive oxidation. The addition of CaO caused the shrinkage during the synthesis and liquid formation during the oxidation above 1673 K (1400 °C). The impurities of CaO and Fe2O3 in the leaching residue can result in an aggressive oxidation at low temperature and a protective oxidation at temperatures above the eutectic point.
Similar content being viewed by others
Introduction
During the secondary melting of aluminum scrap, the molten metal is covered by a salt mixture mainly consisting of NaCl and KCl. After the tapping of aluminum, the salt layer, which has a low amount of metallic aluminum, often termed as “salt cake” consists of the salts, such as NaCl and KCl, and Al2O3, SiO2, MgO, AlN, and metallic Al entrapped in the salt. Recycling of this material is very important from environmental and economic view points as
-
(a)
AlN comes into contact with moisture or rain water hydrolyses and produces NH3 which poses a serious environmental concern.[1–3]
-
(b)
The valuables in the salt cake would be lost unless a suitable strategy for recycling is developed.
The authors of the current study developed a leaching method for the salt cake by saturating the leachant continuously with CO2.[4] They had redesigned the leaching process so that ammonium carbonate/bicarbonate is generated instead of NH3 during the leaching process, which could be used as a synthetic fertilizer. The chemical analysis of a representative sample of the leach residue after carbonic acid leaching[4] is presented in Table I. This analysis does not include the entrapped Al metal which can be as high as 5 wt pct. We could successfully use the leach residue to synthesize oxynitride ceramics.
Oxynitrides ceramics, such as SiAlON and MgAlON, are advanced materials which have received great attention because of their outstanding performance as engineering ceramics, cutting tools, and refractory materials[5,6] for the last 30 years. Different approaches have been used to synthesize oxynitride ceramics from natural and wasted materials other than pure materials.[7–9] As seen in Table I, the leach residue contains, Al2O3, AlN, MgO, SiO2, and small amounts of impurities such as chlorides, fluorides, about 3 wt pct of CaO, and 2 wt pct Fe2O3. The main composition is suitable to synthesize the SiAlON/MgAlON composite, which will be doubly beneficial in terms of reducing the load on the environment as well as value addition with the residue being used as a precursor for the production of oxynitride ceramics.
Oxidation resistance is a significant property for the applications of SiAlON/MgAlON in the refractory field. Hou et al.[10,11] have investigated oxidation behaviors of SiAlON materials and explained the kinetics of oxidation by a series of explicit models developed by Chou.[12] Hou et al.[13] and Ye et al.,[14] reported that synthetic SiAlON/MgAlON from natural material with Fe2O3 impurity would lead to aggressive oxidation. It would be interesting to investigate the performance of the SiAlON/MgAlON material synthesized from the leaching residue of salt cake considering the inherent impurities (Fe2O3 and CaO) present in the residue.
The current study describes the investigation of the high-temperature oxidation of the SiAlON/MgAlON material synthesized from leaching residue of salt cake with CO2-saturated water and synthetic residue with composition corresponding to it. Emphasis is laid on the impacts of Fe2O3 and CaO impurities present in the residue on the oxidation resistance of the ceramic material produced. The presentation of the study is divided into two parts: In Part I, the investigations of the kinetics of oxidation and the activation energies corresponding to the various oxidation stages are presented. The Part II is focused on the structural evaluation and oxidation products to get an insight into the oxidation mechanism.
The main experimental technique employed in Part I was thermo gravimetric analysis (TGA). These experiments were carried out in air under isothermal conditions. The results from the ceramic produced from the leach residue are compared with the corresponding ceramic material produced from the synthetic mixtures to delineate effects of other minor impurities, namely, CaO and Fe2O3, on the product. It is expected that this would enable an understanding of the oxidation resistance of the ceramic composite produced from the salt cake leach residue.
Experimental Procedure
Preparation of the Precursors
Based on the composition of the leaching residue in Table 1, synthetic materials with high purity of Al2O3, SiO2, AlN, MgO, CaCO3, Fe2O3(Fe>69.8 wt pct), Al powder(>99.5 wt pct, <74 μm), Si powder(>99.0 wtpct, <74 μm), and graphite powder were selected. The compositions of three synthetic residues and the leach residue from salt cake are presented in Table II. In this Table, “Pure” refers to the composition with mixtures of pure components. This would correspond to the composition of the leach residue from salt cake without any impurities. “3-Fe2O3” refers to the addition of 3 g. Fe2O3 to the “Pure” sample; “3-CaO” refers to 3 g. CaO (about 5.4 g CaCO3) added to the “Pure” sample. “AD” refers to the residue obtained after the CO2 leaching of the salt cake.
For the synthesis of the ceramic material for the oxidation measurement, the following procedure was adopted. At the outset, 9 g carbon, as a reducing agent, was added to the powder mixtures. Batch compositions were weighed accurately and mixed thoroughly for 8 h in a rotary mill provided with zirconia balls, in ethanol (absolute grade) as the dispersion medium. After the batches were milled, dried powders were uniaxially pressed (from 5 to 7 Mpa) into bars of 60 × 8 × 6 mm and dried at 353 K (80 °C) for 10 hours. Carbothermal reduction and nitridation (CRN)[15,16] technique is adopted as per the following procedure.
The sintering was carried out in two steps. In the first step, the samples, embedded in Si3N4 powder bed were heated to 1873 K (1600 °C) in a MoSi2 furnace and held for 6 hours, under nitrogen (99.99 pct pure) atmosphere (0.1 MPa). In the second step, the samples after sintering in the first step were crushed into powders. 2.5 g metallic Al and 7.5 g metallic Si powders were weighed and added to each 90-g batch, made into bars, and then the same procedure as in the first step is followed.
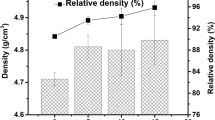
The phases of synthetic samples from the XRD analysis are presented in Table III. The expected presence of Si2Al4O4N4 was observed as a skeletal phase. Besides, 15R-SiAl4O2N4 appears as a minor phase. It is interesting to notice that MgO has apparently reacted with other components in the mixtures during the synthesis, giving rise to Mg0.2Al1.45O2.15N0.15, but disappeared in the case of the AD sample. Traces of Al2O3 and Fe phases have been found in Pure and 3-Fe2O3 samples, respectively. The open porosity and density shows that the sintering process with the presence of CaO can bring densification on the 3-CaO and AD samples.
Oxidation Experiments-Apparatus and Procedure
The kinetics of oxidation of the synthesized samples was investigated by thermogravimetric analysis (TGA) using a SETARM TAG 24 (Setaram instrumentation, Caluire, France) unit which has a balance accuracy of 1 μg. The experimental assembly consists of an electronic microbalance, with the sample and reference hanging in the constant temperature zone of two near-identical chambers in a dual furnace system as shown in Figure 1. During the experiments, a platinum basket containing ceramic sample was hung from one of the balance arms. In the other balance arm, the pure, dehydrated alumina plate sample with nearly the same shape and mass was kept in a similar Pt basket, which served as the reference material for TGA.
The ceramic samples were cut into 6 × 6 × 1 mm coupons and polished. Before oxidation, the pieces were cleaned in toluene, and then in acetone. The sample was held in cross-shaped basket made by platinum wire exposing most of the sample surface to the atmosphere. The oxidation tests were carried out under isothermal mode.
Isothermal oxidation studies were carried out at 1373 K, 1473 K, 1523 K, 1573 K, 1673 K, 1723 K, and 1773 K (1100 °C, 1200 °C, 1250 °C, 1300 °C, 1400 °C, 1450 °C, and 1500 °C) for 6 hours. Before the experiments, the chambers with the sample and reference were purged with purified argon for 12 hours. Argon gas was purified by passing through silica gel and dehydrite (Mg(ClO4)2) to remove moisture and through tube furnaces containing copper and magnesium at 773 K (500 °C) to remove residual oxygen. The furnace was heated from room temperature to the target temperature at a heating rate of 15 K/min under flowing argon. No mass change was observed during the heating period indicating that there was no oxidation.
After the attainment of the desired temperature, argon flow was stopped and the oxidizing gas, viz. air was introduced at a flow rate 0.1 L/min. Preliminary experiments showed that this flow rate was above the starvation rate so that gas phase mass transfer would not be rate-controlling. The mass change during the experiment was recorded every 2 seconds by the computer. After a reaction period of 6 hours, the experiment was terminated by replacing the oxidant gas (air) with argon and cooling down the furnace at the maximum cooling rate allowed for the set-up (40 K/min) until the furnace reached room temperature. Some selected experiments were repeated, and the reproducibility of the results could be confirmed.
The crystalline phases of the samples before and after oxidation were characterized by x-ray diffraction analysis performed on a D8 equipment, from Bruker AXS (Germany). The morphologies of these samples were also studied by SUPRAtm55 field emission scanning electron microscopy. While carrying out the elemental analysis by EDS, the uncertainties in the analysis results of light elements, viz. N and O are taken into account.
Results of the Thermogravimetric Studies
Results of the Isothermal Oxidation Studies
The TGA results obtained in the case of the isothermal oxidation studies are presented in Figures 2(a) through (d). In these figures, the specific mass variation, ΔW/A o (where ΔW represents the mass change recorded, A o is the initial cross section area) was plotted as a function of time at different temperatures.
It is seen that the maximum oxidation level, about 0.07 mg/mm2 was achieved in the cases of the samples marked “Pure” and “3-Fe2O3” (Figures 2(a) and (b)) at 1673 K (1400 °C), in the cases of 3-CaO and AD, even at 1773 K (1500 °C), the oxidation level has reached to more than 0.08 mg/mm2. The difference between them may be contributed by the transformation from Mg0.2Al1.45O2.15N0.15 to 15R-SiAl4O2N4 with more nitrogen content dissolved in the solid solution. In general, at lower temperatures, viz. 1373 K and 1473 K (1100 °C and 1200 °C), all of the four samples show better oxidation resistance beyond 5000 seconds. This is particularly evident in the case of the AD sample at 1473 K (1200 °C), the reaction is very fast in the beginning and then coming to a sharp change to a very slow rate after 2000 seconds. Besides that, there are some interesting observations, in the cases of the AD precursor, at 1573 K and 1673 K (1300 °C and 1400 °C), the oxidation rate did not show an increase beyond the initial period of increasing rate. A similar behavior is observed in the case of the sample marked “Pure” at 1773 K (1500 °C). This behavior may be attributed to the formation of a barrier layer even during the ramping which would hinder further oxidation.
Oxidation Products Analysis
The samples after oxidation at different temperatures were taken out and examined by x-ray diffraction (XRD) and scanning electron microscopy (SEM) coupled with electron dispersive spectroscopy (EDS). A detailed presentation of all XRD patterns and the micrographs is shown in Part II of this study, presented as a separate article, wherein, as stated earlier, the mechanism of the oxidation process is examined on the basis of the structure evolution. This division of the presentation is partly due to the extensive data generated in the current study and partly to provide clarity for the reader in the presentation of the results.
The major compounds identified in the products after the oxidation at different temperatures are presented in Table IV.
The microstructures of the oxide scale at low-magnification corresponding to the four samples after oxidation in air for 6 hours obtained by SEM are presented in Figure 3.
Pure (Figure 3(a)) and 3-Fe2O3 (Figure 3(b)) samples contain apparently only one type of crystals, viz. mullite (3Al2O3·2SiO2). The product layer in the case of the AD sample consists of a mixture of two types of crystals according to EDS analysis in Table V, elongated mullite (spot 2) and cubic grains (MgAl2O4) (spot 1) embedded in a matrix that seems to be vitreous. Besides, some large grains exhibiting corundum phase (Al2O3) (spot 3) were found to be present in the oxidation product of 3-CaO sample (Figure 3(c)). The important difference between Figure 3(a), (b) and (c), (d) is that the oxide scales in 3-CaO (Figure 3(c)) and AD (Figure 3(d)) have a much more glassy appearance. As can be seen, these two oxide scales exhibit “droplet” appearance with different compositions. The liquid phase in AD sample (spot 5) contains iron in addition to the other components present in the 3-CaO sample (spot 4). The droplets are likely to originate from the solidification of liquid phase formed at the oxidation temperature of 1673 K (1400 °C).
Discussion
Thermodynamic Aspects
The oxidation of the precursors prepared in the current study is a very complex process involving the formation of a variety of products like spinel, mullite, etc. The basic reaction, in a simplified form, can be written as
when z = 3.13, ΔH 1 at 1043 K (770 °C) for reaction [1] = −1974.9 ± 11.7 kJ/mol, where ΔH 1 is the reaction enthalpy of oxidative drop solution.
When z = 3.6, ΔH 1 at 1043 K (770 °C) for reaction [1] = −1737.8 ± 6.8 kJ/mol[17]
In the case of synthetic samples in this article, z = 4, ΔH 1 would be reasonably close to the value of −1737.8 kJ/mol.
In the case of the compound, Mg0.2Al1.45O2.15N0.15, the oxidation reaction can be represented as
The oxidation of SiAlON/MgAlON is most likely to be an exothermic reaction (although the enthalpy data for the oxidation of MgAlON could not be found in the literature by the present authors). Consequently, the energy released in a small region would cause a local increase in temperature. In addition, the kinetics of solid-state reactions are known[18] to be sensitive to the size of crystals, oxidant transportation, and many other factors which are likely to change during the process. The relative contributions to the overall reaction rate would be to make the effective activation energy dependent on the extent of oxidation.[19] In the current discussions, a step modeling method was adopted to extract the effective activation energy corresponding to the various oxidation stages.
Isothermal Oxidation
As the flow rate of the oxidant gas is more than the starvation rate, in the present analysis, the gas phase mass transfer is not considered as a rate-determining step.
Initial linear region
In the analysis of gas–solid reactions, the linear rate law is generally valid in the initial stage of the oxidation process. This would indicate that the rate-controlling reaction would be the chemical reaction as the surface is exposed directly to the oxidant gas. Equation [3] would represent the chemical reaction as the rate-controlling step.[20]
where k is the rate constant, b c is an additive constant (ideally equal to zero).
Figure 4 shows the linear fits for the first 1000 seconds oxidation of Pure and 3-Fe2O3 samples, the oxidation data are plotted as ΔW/A o vs t. The curves are in general linear indicating that the chemical reaction is the rate-controlling step at this stage. A closer look at the curves indicates that these apparently linear plots have a slight upward curvature. This is more apparent with the plot at 1573 K (1300 °C). One plausible explanation is the existence of local nonisothermal conditions on the surface of the sample. As reactions are exothermic, there is a likelihood of a nonisothermal situation with the increase of local temperature on the surface of the sample, which could accelerate the oxidation. The ceramic samples are expected to exhibit a low thermal conductivity, and consequently, the heat transfer within the sample would be slow resulting in the increase of local temperature. This situation is further enhanced by the oxidation with the formations of spinel and mullite phases, which are both exothermic reactions.
The nonlinear region
In order to identify the diffusion part of the oxidation reaction as the oxidized layer begins to grow, the oxidation data are plotted as (ΔW/A o)2 vs t in Figure 5. For convenience, the chemical reaction controlling part (the first 1000 seconds) is included as a starting point.
The squared specific weight gain vs t for different types of oxidation kinetics A: arctan-rate law, P: parabolic law, t o: t o is the time at which the crystallization process reaches steady state. Note: P pore: diffusion through pores, P liquid: diffusion through liquid, symbol line: experimental data, solid line: fitted line based on different kinetics
According to the investigation of Persson et al.,[21] normally isothermal diffusion kinetics was classified into four different types. In the current study, the same classification is followed.
In the first type, the addition of CaO during the synthesis leads to the shrinkage of the synthetic sample with lesser porosity which would greatly improve the oxidation resistance. At lower temperatures, viz. 1473 K and 1523 K (1200 °C and 1250°C), 3-CaO oxidation is controlled by diffusion obeying the parabolic law.
The second type, for 1673 K (1400 °C) of 3-Fe2O3 and Pure, derivative of (ΔW/A o)2 decreases with increasing time during the entire experiment. The third type, in the case of the sample AD, at 1473 K (1200 °C), the slope of (ΔW/A o)2 increases at the outset, and finally follows the parabolic rate law behavior. It is necessary to develop a combination of these kinetics models (the first type and second type) to explain the oxidation behavior in the cases of the oxidation of 3-Fe2O3, Pure, and AD samples at lower temperatures. In the case of the fourth type, once liquid is formed, the obtained oxidation curve for sample marked “Pure” at 1773 K could be described by the parabolic rate law. This is also applicable in the cases of 3-CaO at 1673 K and 1773 K (1400 °C and 1500 °C) and in the cases of the AD sample at 1573 K, 1673 K, and 1773 K (1300 °C, 1400 °C, and 1500°C). In fact, there is actually even a fifth type, in the case of the oxidation of the 3-CaO sample at 1573 K (1300 °C), which can be considered as the combination of the second and fourth type, once the liquid is formed during the oxidation.
If oxide products crystallize gradually after the chemical reaction-controlled oxidation region, the effective diffusion rate is time-dependently decreasing because of the decrease in the cross section available for diffusion. The oxidation kinetics can be considered to follow an arctan-rate law of the form[21–27]:
where ΔW/A o is the specific mass variation (weight change per surface area), k p is the parabolic-rate constant, β is the rate constant for the decrease of the cross-section area, f is the fraction of original area remains amorphous, t o is the time at which the crystallization process reaches steady state (cross-sectional area becomes constant), and d is an additive constant (in this case, it is the mass variation at around 1000 seconds). At t o in Figure 5, corresponding to the oxidation of the AD sample at 1473 K (1200 °C), the oxidation kinetics would change, and the arctan-rate law is no longer obeyed. Consequently, after t o, the effective diffusion rate would be parabolic, assuming that the oxide scale is protective[21]:
where \( k_{{\rm p}}^{\rm o} \) is an apparent rate constant, and b o is an additive constant.
Figure 5 shows the curves obtained by square fitting the combination of the arctan and parabolic-rate laws in the case of 1473 K (1200 °C)-AD, parabolic-rate law in the cases of 3-CaO and 1773 K (1500 °C)-Pure. It can be seen that the equations fit the experimental results quite well supporting the validity of the proposed combinations of rate laws.
Activation energies from isothermal experiments
The rate constants determined from the results shown in Figures 4 and 5 for the linear (k), arctan (k p ), parabolic (\( k_{\rm p}^{\rm o} \)) regimes, respectively, are shown in the Arrhenius-type plots of Figure 6. The activation energies (E α) for the oxidation can be then determined by fitting the Arrhenius equation in Eq. [6]:
where A is the pre-exponential factor, R is the universal gas constant (8.314 J/mol K), and T is the absolute temperature. The best fits lines are presented in Fig. 6, from which the activation energies were obtained.
The Arrhenius plot of the rate constants for the linear (a), and arctan and parabolic fit (b) oxidations of four different samples in air in the temperature range 1373 K to 1773 K (1100 °C to 1500 °C). The lines are linear fits of the point data. ■--- 3-Fe2O3-linear, □--- Pure-linear, ▲--- 3-CaO-linear, ■--- 3-Fe2O3-Arctan, □--- Pure-Arctan, ○--- 3-CaO-Parabolic, liquid ●--- AD-Parabolic, liquid, △--- 3-Fe2O3-Parabolic, pore diffusion, ▲--- 3-CaO-Parabolic, pore diffusion, ★--- Pure-Parabolic, pore diffusion, ◆--- AD-Arctan
The E a, for the \( k_{\rm p}^{\rm o} \) (parabolic rate constant) essentially represents the activation energy for the diffusion, whereas E α, for the k p (the rate constant derived from arctan relationship) and k (chemical reaction rate constant) represent the activation energy with arctan rate-controlling and chemical-reaction controlling, respectively. It can be concluded from the values (Figure 6; Table VI) determined for the linear (k), arctan (k p), and parabolic (\( k_{\rm p}^{\rm o} \)) regimes as follows:
-
i.
The activation energy for chemical reaction for Pure sample (174 kJ/mol) is much lower than that of the sample with CaO addition: 3-CaO (248.20 kJ/mol), but comparable with the literature value for β-SiAlON powders[28] (161 kJ/mol). Normally, the sintered samples with sintering additives have a higher value of activation energy. It is suggested that facilitation on the crystal growth, the transformation from Mg0.2Al1.45O2.15N0.15 to 15R-SiAl4O2N4 with more content of N or the reduction of lattice defects during the sintering may benefit the oxidation resistance.
-
ii.
The sample with addition of Fe2O3, i.e. 3-Fe2O3 is less oxidation resistant than Pure in the entire temperature zone (higher k p), with relatively lower E a values (141.16 kJ/mol and 314.78 kJ/mol) for linear and arctan of 3-Fe2O3, respectively, compared to those for Pure (174.88 kJ/mol and 361.31 kJ/mol), is likely to be indicative of a lattice distortion making the sample vulnerable to oxidation.[14] The large value of activation in arctan region indicated that the rate-controlling mechanism is rather outward diffusion of Al3+, Si4+ cations from the intergranular phase into the oxide scale for compensating the crystallization.[29,30]
-
iii.
The activation energy associated with the \( k_{\rm p}^{\rm o} \) values of 3-CaO, viz. 242.83 kJ/mol is higher than that obtained from 3-Fe2O3 at lower temperatures (142 kJ/mol). The low activation energy may be attributed to the lattice distortion mentioned earlier, which may result in improved transport of oxygen and nitrogen.
-
iv.
At higher temperatures, the activation energy associated with the \( k_{\rm p}^{\rm o} \) of 3-CaO is much larger than that at lower temperatures (398 kJ/mol vs 242.83 kJ/mol, respectively), revealing a difference in the diffusion-controlling mechanisms. The transformation of oxygen state form molecules to ions has always been proved to accompany the changing of the activation energy for diffusion.[29,31,32] The much higher activation energy is ascribed to diffusion of O2− ions from the liquid phase into the oxide scale. The moderate value of the activation energy in the latter case is due to the inward diffusion of oxygen gas molecules through pores.
-
v.
Owing to properties of AD samples, above 1573 K (1300 °C), a barrier layer can be formed during the heating, where the oxidation has not even started yet. It was inferred that, as a consequence, only the E a associated with the \( k_{\rm p}^{\rm o} \) at higher temperatures could be estimated. In other words, the rate-controlling step for the oxidation of AD is the diffusion through liquid above 1573 K (1300 °C). The lnk p of 1473 K (1200 °C)-AD has the highest value which indicates that the transition metals like Fe or FeO x , (which may dissolve into the lattice of SiAlON) can have an accelerated effect on the oxidation rate.
Conclusions
The effects of the additives (Fe2O3 or CaO) on the oxidation of SiAlON/MgAlON samples synthesized in the current study were investigated by TGA technique under the isothermal mode. The oxidations of four different samples, viz. Pure, 3-Fe2O3, 3-CaO, and AD in air were investigated in the temperature range from 1373 K to 1773 K (1100 °C to 1500 °C). Based on this study, the following conclusions can be drawn:
(1) The oxidation processes of “Pure” and 3-Fe2O3 at these temperatures are passive and have a three stage kinetics that first obeys the linear law, then arctan-rate law, then followed by the parabolic-rate law below 1773 K (1500 °C). This complex oxidation kinetics reflects the crystallization of the oxide scales, and their protective character.
(2) The addition of Fe2O3 caused lattice distortion, which is believed to result in a lower activation energy for aggressive oxidation in linear region and diffusion region compared with Pure and 3-CaO.
(3) The addition of CaO content resulted in a combined diffusion mechanism: diffusion of oxygen molecule through pores at lower temperatures (due probably to the shrinkage of sample 3-CaO during the synthesis) and diffusion of oxygen ions inward through formed liquid at higher temperatures.
(4) SiAlON synthesized from salt cake shows a combined oxidation character: the impurities of CaO and Fe2O3 in the sample AD can result in an aggressive oxidation at low temperature [1473 K (1200 °C)] and a protective oxidation at the temperatures above lower eutectic point [1573 K (1300 °C)].
References
Rao, S.R.: Resource recovery and recycling from metallurgical wastes. 1st ed., Elsevier, The Netherlands, 2006.
H. Shen, E. Forssberg: Waste Manage., 2003, vol. 23, pp. 942–44.
O. Manfredi, W. Wuth, I. Bohlinger: JOM, 1997, vol. 49, pp. 48–51.
P. Li, M. Guo, M. Zhang, L.D. Teng, S.Seetharaman: Metall. Mater. Trans. B., 2012, vol. 43B, pp. 1220–30.
I.-W. Chen and A. Rosenflanz: Nature, 1997, vol. 389, pp. 701–04.
T. Ekström and M. Nygren: J. Am. Ceram. Soc., 1992, vol. 75, pp. 259–76.
A.D. Mazzoni and E.F. Aglietti: Appl. Clay Sci., 1998, vol. 12, p. 447.
F.J. Li, T. Wakihara, T. Wakihara, J. Tatami, K. Komeya, T. Meguro, and K.J.D. MacKenzie: J. Am. Ceram. Soc., 2007, vol. 90, pp. 1541–44.
J. Wang, N. Li, C.C Shi and C.C. Jia: J. Univ. Sci. Technol. Beijing, 2000, vol. 7, p. 209.
X.M. Hou, K.C. Chou, X.J. Hu, and H.L. Zhao: J. Alloys. Compd., 2008, vol. 459, p.123.
X.M. Hou, G.H. Zhang, and K.C. Chou: Int. J. Appl. Ceram. Technol., 2008, vol. 5, p. 529.
K.C. Chou: J. Am. Ceram. Soc., 2006, vol. 89, p. 1568.
X.M. Hou, C.S. Yue, M. Zhang, and K.C. Chou: Int. J. Min. Met. Mater., 2010, vol. 18, p. 77.
G.T. Ye, J.L. Shang, D.G. Zhang, M. Liang, and Y.S Chen: J. Am. Ceram. Soc., 2010, vol. 93, pp. 322–25.
W. Dai, W. Lin, A. Yamaguchi, J. Ommyoji, J. Yu, and Z. Zou: J. Ceram. Soc. Jpn., 2007, vol. 115, pp. 42–6.
I. J. Davies, T. Minemura, N. Mizutani, M. Aizawa, and K. Itatani: J. Mater. Sci., 2001, vol. 36, pp. 165–72.
J.J. Liang, A. Navrotsky, V.J. Leppert, M.J. Paskowitz and S. H. Risbud: J. Mater. Res., 1999, vol. 14, pp. 4630–36.
S. Vyazovkin and C.A. Wight: Phys. Chem., 1997, vol. 101, pp. 8279.
M. Maciejewski: J. therm. Anal., 1992, vol. 38, pp. 51.
A. Bellosi, E. Landi and A. Tampieri: J. Mater. Res., 1993, vol. 8, p. 565.
J. Persson and M. Nygren: J. Eur. Ceram. Soc., 1994, vol. 13, no.5, pp. 467–84.
K.G. Nickel: Corrosion of Advanced Ceramics: Measurement and modelling. NATO ASI Series, Series E (Applied Sciences), vol. 267, 1994.
L.O. Nordberg, M. Nygren, P.O. Käll and Z.J. Shen: J. Am. Ceram. Soc., 1998, vol. 81, pp. 1461–70.
L.O. Nordberg, P.O. Käll, and M. Nygren: Key Eng. Mater., 1995, vol. 113, pp. 39–48.
J. Persson, P.O. Käll, and M. Nygren: J. Eur. Ceram. Soc., 1993, vol. 12, pp. 177–84.
J. Persson, T. Ekstrom, P. O. Käll and M. Nygren: J. Eur. Ceram. Soc., 1993, vol. 11, pp. 363–73.
J. Persson, P.O. Käll and M. Nygren: J. Am. Ceram. Soc., 1992, vol. 75, pp. 3377–84.
K.J.D. MacKenzie, S. Shimada, and T. Aoki: J. Mater. Chem., 1997, vol. 7, pp. 527–30.
J. A. Costello and R. E. Tressler: J. Am. Ceram. Soc., 1986, vol.69, pp.674-681.
T. Chartier, J. M. Laurent, D. S. Smith, F. Valdivieso, P. Goeuriot, and F. Thevenot: J. Mater. Sci., 2001, vol. 36, pp. 3793–800.
Z. Zheng, R.E. Tressler, and K.E. Spear: J. Electrochem. Soc., 1990, vol. 137, pp. 2812–16.
T. Narushima, T. Goto, and T. Hirai: J. Am. Ceram. Soc., 1989, vol. 72, pp. 1386–90.
Acknowledgments
The authors would like to thank the National Science Foundation of China for the financial support (Nos. 51072022, 51074009, 50874013), under the Program for Talents youth in USTB (FRF-TP-09-005B). The supply of salt cake by Stena Metall AB is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted May 17, 2012.
Rights and permissions
About this article
Cite this article
Li, P., Zhang, M., Teng, L. et al. Oxidation Studies of SiAlON/MgAlON Ceramics with Fe2O3 and CaO Impurities, Part I: Kinetics. Metall Mater Trans B 44, 210–219 (2013). https://doi.org/10.1007/s11663-012-9763-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9763-y