Abstract
Objective
To investigate the effect of Xuezhikang (血脂康, XZK) on renal cell apoptosis in diabetic rats and the possible mechanism.
Methods
Sixty-six rats were randomly divided into 3 groups: the normal, model and XZK groups. In each group, the rats were further randomly divided into 3-month and 6-month subgroups, respectively. Diabetes of rats was induced by a single intraperitoneal injection of 1% streptozocin at 60 mg/kg body weight. Rats in the XZK group received gastric perfusion of XZK (1200 mg/kg body weight) everyday for 3 or 6 months, while rats in the normal and model groups received equal volume of saline. Twenty-four hours’ urine was collected for urinary albumin excretion (UAE) measurement. Periodic acid-Schiff (PAS) and Masson’s trichrome staining were used for saccharides and collagen detection. Cell apoptosis of renal cortex was investigated by TdT-mediated dUTP nick end labeling (TUNEL) staining. Bax and Bcl-2 expressions were detected by immunohistochemistry and Western blot, respectively. Cytochrome C (Cyt C) and caspase-9 concentration were detected by Western blot.
Results
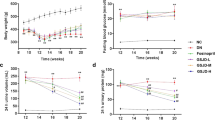
Compared with the model group, XZK treatment could significantly decrease the kidney hypertrophy index, 24 h UAE, renal cell apoptosis, cytoplasmic Cyt C level and active caspase-9 level, as well as suppress the increment of Bax and up-regulate the expression of Bcl-2, leading to the suppression of Bax/Bcl-2 ratio at 3 and 6 months (P<0.05 or P<0.01). Moreover, XZK treatment could alleviate the deposition of PAS-stained saccharides and Masson’s trichromestained collagen to different extent.
Conclusions
Renal cell apoptosis was observed in diabetic kidney, in which mitochondrial apoptotic pathway might be involved. XZK treatment could attenuate pathological changes in diabetic kidney and reduce renal cell apoptosis, probably via the suppression of Bax/Bcl-2 ratio, which lead to inhibition of Cyt C release and following caspase-9 activation.
Similar content being viewed by others
References
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053.
Yang WY, Lu JM, Weng JP, Jia WP, Ji LN, Xiao JZ, et al. China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in china. N Engl J Med 2010;362:1090–1101.
Bloomgarden ZT. Diabetic nephropathy. Diabetes Care 2008;31:823–827.
White KE, Bilous RW. Structural alterations to the podocyte are related to proteinuria in type 2 diabetic patients. Nephrol Dial Transplant 2004;19:1437–1440.
Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem 2004;259:67–70.
Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucoseinduced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006;55:225–233.
Verzola D, Gandolfo MT, Ferrario F, Rastaldi MP, Villaggio B, Gianiorio F, et al. Apoptosis in the kidneys of patients with type ? diabetic nephropathy. Kidney Int 2007;72:1262–1272.
Danesh FR, Kanwar YS. Modulatory effects of HMG-CoA reductase inhibitors in diabetic microangiopathy. FASEB J 2004;18:805–815.
Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006;17:2006–2016.
Kou W. A review of basic and clinical research of Xuezhikang. Chin J Intern Med (Chin) 1998;37:364–366.
Wang F, Wu HM. Xuezhikang for diabetic kidney disease: a systematic review of randomized controlled trials. Chin J Evid Based Med (Chin) 2009;9:63–70.
Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. Simvastatin induces activation of the serine-threonine protein kinase AKT and increases survival of isolated human pancreatic islets. Transplantation 2002;74:1063–1069.
Lin CL, Cheng H, Tung CW, Huang WJ, Chang PJ, Yang JT, et al. Simvastatin reverses high glucose-induced apoptosis of mesangial cells via modulation of Wnt signaling pathway. Am J Nephrol 2008;28:290–297.
Piconi L, Corgnali M, Da Ros R, Assaloni R, Piliego T, Ceriello A. The protective effect of rosuvastatin in human umbilical endothelial cells exposed to constant or intermittent high glucose. J Diabetes Complicat 2008;22:38–45.
Li H. Pathogenesis and therpaeutit targets of diabetic microvascular complications. J Zhejiang Univ (Med Sci, Chin) 2006;39:233–237.
Mogensen CE. Microalbuminuria as a predictor of clinical diabetic nephropathy. Kidney Int 1987;31:673–689.
Basi S, Fesler P, Mimran A, Lewis JB. Microalbuminuria in type 2 diabetes and hypertension: a marker, treatment target, or innocent bystander? Diabetes Care 2008;31:5194–5201.
Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11.
Fried LF. Effects of HMG-CoA reductase inhibitors (statins) on progression of kidney disease. Kidney Int 2008;74:571–576.
Eller P, Eller K, Wolf AM, Reinstadler SJ, Tagwerker A, Patsch JR, et al. Atorvastatin attenuates murine antiglomerular basement membrane glomerulonephritis. Kidney Int 2010;77:428–435.
Lundberg S, Lundahl J, Gunnarsson I, Jacobson SH. Atorvastatin-induced modulation of monocyte respiratory burst in vivo in patients with IgA nephropathy: a chronic inflammatory kidney disease. Clin Nephrol 2010;73:221–228.
Chade AR, Zhu XY, Grande JP, Krier JD, Lerman A, Lerman LO. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J Hypertens 2008;26:1651–1660.
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome C release from mitochondria. Cell Death Differ 2006;13:1423–1433.
Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, et al. Cytochrome C deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 2000;101:389–399.
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that results in the release of cytochrome C. Cell Death Differ 2000;7:1166–1173.
Cheng Y, Gulbins E, Siemen D. Activation of the permeability transition pore by Bax via inhibition of the mitochondrial BK channel. Cell Physiol Biochem 2011;27:191–200.
Voehringer DW, McConkey DJ, McDonnell TJ, Brisbay S, Meyn RE. Bcl-2 expression causes redistribution of glutathione to the nucleus. PNAS 1998;95:2956–2960.
Kang BP, Frencher S, Reddy V, Kessler A, Malhotra A, Meggs LG. High glucose promotes mesangial cell apoptosis by oxidant-dependent mechanism. Am J Physiol Renal Physisol 2003;284:F455–F466.
Jung DS, Li JJ, Kwak SJ, Lee SH, Park J, Song YS, et al. FR167653 inhibits fibronectin expression and apoptosis in diabetic glomeruli and in high-glucose-stimulated mesangial cells. Am J Physiol Renal Physiol 2008;295:F595–F604.
Sohn E, Kim J, Kim CS, Kim YS, Jang DS, Kim JS. Extract of the aerial parts of Aster koraiensis reduced development of diabetic nephropathy via anti-apoptosis of podocytes in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun 2010;391:733–738.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Wn., Zheng, Fp., Lai, Dw. et al. Xuezhikang (血脂康) reduced renal cell apoptosis in streptozocin-induced diabetic rats through regulation of Bcl-2 family. Chin. J. Integr. Med. 22, 611–618 (2016). https://doi.org/10.1007/s11655-015-2050-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-015-2050-4