Abstract
Objective
To investigate the effect of Dan-gua Fang (丹瓜方) on adenosine 5′-monophosphate (AMP) activated protein kinase (AMPK) α expression in liver and subsequent improvement of glucose and lipid metabolism.
Methods
Forty 13-week-old diabetic Goto-Kakizaki (GK) rats were randomly divided into model, Dan-gua Fang, metformin and simvastatin groups (n=10 for each), and fed high-fat diet ad libitum. Ten Wistar rats were used as normal group and fed normal diet. After 24 weeks, liver expression of AMPKα mRNA was assessed by real-time PCR. AMPKα and phospho-AMPKα protein expression in liver was evaluated by Western blot. Liver histomorphology was carried out after hematoxylin-eosin staining, and blood glucose (BG), glycosylated hemoglobin A1c (HbA1c), food intake and body weight recorded.
Results
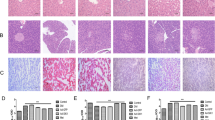
Similar AMPKα mRNA levels were found in the Dan-gua Fang group and normal group, slightly higher than the values obtained for the remaining groups (P<0.05). AMPKα protein expression in the Dan-gua Fang group animals was similar to other diabetic rats, whereas phospho-AMPKα (Thr-172) protein levels were markedly higher than in the metformin group and simvastatin group (P<0.05), respectively. However, phosphor-AMPKα/AMPKα ratios were similar in all groups. Dan-gua Fang reduced fasting blood glucose with similar strength to metformin, and was superior in reducing cholesterol, triglycerides, high-density lipoprotein cholesterol as well as improving low-density lipoprotein cholesterol in comparison with simvastatin and metformin. Dan-gua Fang decreases plasma alanine aminotransferase (ALT) significantly.
Conclusion
Dan-gua Fang, while treating phlegm-stasis, could decrease BG and lipid in type 2 diabetic GK rats fed with high-fat diet, and effectively protect liver histomorphology and function. This may be partly explained by increased AMPK expression in liver. Therefore, Dan-gua Fang might be an ideal drug for comprehensive intervention for glucose and lipid metabolism disorders in type 2 diabetes mellitus.
Similar content being viewed by others
References
McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7–18.
Yki-Jarvinen H. Management of type 2 diabetes mellitus and cardiovascular risk: lessons from intervention trials. Drugs 2000;60:975–983.
Group AS, Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–828.
Group AS, Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574.
Group AS, Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, et al. Effects of intensive bloodpressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585.
Yang LQ, Huang SP, Heng XP, He WD, Zhou GY. Clinical observation of Dangua prescription on type 2 diabetes patients with long-term hyperglycosemia. J Fujian Univ Tradit Chin Med (Chin) 2010;20:3–6.
Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 2009;196:81–98.
Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, et al. The creb coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 2005;437:1109–1111.
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414:799–806.
Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, et al. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 2005;54:1331–1339.
Corton JM, Gillespie JG, Hardie DG. Role of the AMPactivated protein kinase in the cellular stress response. Curr Biol 1994;4:315–324.
Henin N, Vincent MF, Gruber HE, Van den Berghe G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J 1995;9:541–546.
Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, et al. Inhibition of lipid synthesis through activation of AMP kinase: An additional mechanism for the hypolipidemic effects of berberine. J Lipid Res 2006;47:1281–1288.
Velasco G, Geelen MJ, Guzman M. Control of hepatic fatty acid oxidation by 5′-AMP-activated protein kinase involves a malonyl-CoA-dependent and a malonyl-CoA-independent mechanism. Arch Biochem Biophys 1997;337:169–175.
Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 2001;291:2613–2616.
Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab 2005;289:E794–E800.
Muoio DM, Seefeld K, Witters LA, Coleman RA. AMPactivated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: Evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J 1999;338(Pt 3):783–791.
Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 2008;57:306–314.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174.
El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 2000;275:223–228.
Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 2006;281:2654–2660.
Wang X, Zhou L, Shao L, Qian L, Fu X, Li G, et al. Troglitazone acutely activates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Life Sci 2007;81:160–165.
Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMPactivated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun 2004;314:580–585.
Sun W, Lee TS, Zhu M, Gu C, Wang Y, Zhu Y, et al. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation 2006;114:2655–2662.
Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 2004;10:727–733.
Hardie DG. Minireview: the AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology 2003;144:5179–5183.
Heng XP, Chen KJ, Hong ZF, He WD, Chu KD, Chen WL, et al. Glucose endothelial cytotoxicity and protection by Dan-gua Fang, a Chinese herb prescription in HUVEC in hyperglycemia medium. J Diabet Complications 2009;23:297–303.
Heng XP, Chen KJ, Hong ZF, He WD, Chu KD, Chen WL, et al. Research on ROS content variance of inner-ECV 304 in high glucose medium and inflence of Dan-gua Fang. Guangming J Chin Med (Chin) 2008;23:551–555.
Heng XP, Chen KJ, Hong ZF, He WD, Chu KD, Chen WL, et al. Anticolchicine cytotoxicity enhanced by Dan-gua Fang, a chinese herb prescription in ECV 304 in mediums. Chin J Integr Med 2011;17:126–133.
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998;114:842–845.
Zhang ZF, Zhao G, Zhu Y, Wang LX, Zhu L. Efficacy of metformin in the treatment of adult nonalcoholic fatty liver disease: a meta-analysis. World Chin J Dig (Chin) 2010;18:1717–1723.
Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 2004;126:1287–1292.
Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology 2005;41:690–695.
Yuan XW, Feng WH, Tong GY, Zhu DL. Effect of atorvastatin on nonalcoholic steatohepatitis induced by high-fat diet in rats. Jiangsu Med J (Chin) 2011;11:1247–1249.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 81173179), the Natural Science Foundation of Fujian Province (No. 2011J01198), the Fujian Medical Innovation Project (No. 2009-CX-19), the Research Foundation of Fujian Health Department (No. Zlcnfm02), the Fujian Provincial Department of Education Category A Projects (No. JA09131), the Fujian Health Department of Traditional Chinese Medicine Research (No. WZY0920), the CHEN Ke-ji Integrative Medicine Development Fund (No. CKJ2008047, CKJ2009004), and the Integrative Medicine of Fujian Key Laboratory of Age-related Diseases Funded Projects (No. 2008J1004-10)
Rights and permissions
About this article
Cite this article
Lan, Yl., Huang, Sp., Heng, Xp. et al. Dan-gua Fang (丹瓜方) improves glycolipid metabolic disorders by promoting hepatic adenosine 5′-monophosphate activated protein kinase expression in diabetic Goto-Kakizaki rats. Chin. J. Integr. Med. 21, 188–195 (2015). https://doi.org/10.1007/s11655-014-1826-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-014-1826-2