Abstract
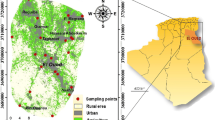
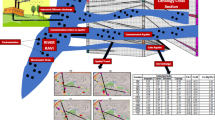
Piper (1944) diagram has been the basis for several important interpretations of the hydrogeochemical data. As seen in this diagram, most natural waters contain relatively few dissolved constituents, with cations (metals or bases) and anions (acid radicles) in chemical equilibrium with one another. Apart from the facies representation, the composition of the mixed sample can be identified in terms of the composition of the parental solution. To bring out this advantage of the Piper diagram, a study was conducted in the Kalpakkam region of Tamilnadu, South India. By taking the geology and water table into consideration, two sample locations were selected as parent solution and third one as the mixture sample. All three samples were analyzed for calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), chloride (Cl), sulphate (SO4) and phosphate (PO4) by Ion Chromatograph (Metrohm IC 861). HCO3 was determined by volumetric titration. The Piper diagram shows that parent solutions clustered towards Na-Mg-Ca-HCO3-Cl and Na-HCO3 facies, and the mixing sample belongs to Na-Mg-HCO3 facies. Phreeqc interactive (Ver 2.8) along with the original composition of the mixture sample was used to correlate the mixing proportion identified by the Piper diagram.
Similar content being viewed by others
References
APHA (1998) Standard Method for the Examination of Water and Wastewater [M]. APHA, Washington DC, USASS, 19th edition.
Arumugham V. (1994) Site Characterization for Location of Radioactive Waste Respository—A Case Study [M]. Ph.D Thesis. Indian Institute of Technology, Bombay, India.
Barut I.F., Erdogan N., and Basak E. (2004) Hydrogeochemical evaluation of Western Anatolian mineral waters [J]. Environmental Geol. 45, 494–503. DOI 10.1007/s00254-003-0904-0.
Benony K., Kortatsi Collins K. Tay, Geophrey Anornu, Ebenezer Hayford, Grace A., and Dartey (2008) Hydrogeochemical evaluation of groundwater in the lower Offin basin, Ghana [J]. Environ. Geol. 53, 1651–1662. DOI 10.1007/s00254-007-0772-0.
Byoung-Young Choi., Seong-Taek Yun., Soon-Young Yu., Pyeong-Koo Lee., Seong-Sook Park., Gi-Tak Chae., and Bernhard Mayer (2005) Hydrochemistry of urban groundwater in Seoul, South Korea: Effects of land-use and pollutant recharge [J]. Environ. Geol. 48, 979–990. DOI 10.1007/s00254-004-1205-y.
Chidambaram S., Ramanathan A.L., Prasanna M.V., Loganathan D., Badrinarayanan T.S., Srinivasamoorthy K., and Anandhan P. (2008) Study on the impact of tsunami on shallow ground water from portnova to pumpuhar, using geoelectrical technique-south east coast of India [J]. Indian Journal of Marine Sciences. 37(20), 121–131.
Cruz J.V. and Amaral C.S. (2004) Major ion chemistry of groundwater from perched-water bodies of the Azores (Portugal) volcanic archipelago [J]. Appl. Geochem. 19, 445–459.
Domenico P.A. and Schwartz F.W. (1998) Physical and Chemical Hydrogeology (2nd ed.) [M]. pp.506. Wiley, New York.
Garrels R.M. and Mackenzie F.T. (1967) Origin of the chemical compositions of some springs and lakes. In Equilibrium Concepts in Natural Water Systems (ed. Gound R.F.) [M]. American Chemical Society Publications, Washington.
Helena B.A. (1998) A case of hydrochemical characterization of an alluvial aquifer influenced by human activities [J]. Water Air Soil Pollut. 112, 365–387.
Piper A.M. (1944) A graphic procedure in the geochemical interpretation of water analysis [M]. Trans. AM Geophys. Union. 25, 914–923.
Piper A.M. (1953) A graphic procedure in the geochemical interpretation of water analyses [M]. U.S. Geol. Survey, Groundwater. No. 12.
Prasanna M.V., Chidambaram S., Shahul Hameed A., and Srinivasamoorthy K. (2009) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data [J]. Environ. Monit. Assess. 168, 63–90.
Renick B.C. (1925) Base exchange in ground-water by silicates as illustrated in Montana [M]. U.S. Geol. Surv., W-S. Paper. 520, 53–72.
Roger Revelle (1941) Criteria for recognition of sea-water in ground-water [M]. pp.595–596. Trans. Amer. Geophys. Union (part III).
Singh V.S. and Saxena V.K. (2004) Assessment of utilization groundwater resources in a coastal shallow aquifer. In Proceeding of the 2nd Asia Pacific Association of Hydrology & Water Resources Conferences [M]. Singapore. Vol. II, 357–364.
Singh V.S., Saxena V.K., Prakash B.A., Mondal N.C., and Jain S.C. (2004) Augmentation of Groundwater Resources in Saline Ingress Coastal Deltaic Area [M]. pp.1–61. NGRI-Tech. Report. No. NGRI-2004-GW-422.
Srinivasamoorthy K., Chidambaram S., Prasanna M.V., Vasanthavihar M., John Peter., and Anandhan P. (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain-A case study from Mettur taluk, Salem District, Tamil Nadu, India [J]. J. Earth System Sci. 117(1), 49–58.
Sukhija B.S., Reddy D.V., and Nagabhushaman P. (1998) Isotopic fingerprints of paleoclimates during the last 30000 years in deep confined groundwaters of southern India [J]. Quartern Res. 50, 252–260.
Wen X., Wu Y., Su J., Zhang Y., and Liu F. (2005) Hydrochemical characteristics and salinity of groundwater in the Ejina Basin, Northwestern China [J]. Environ. Geol. 48, 665–675. DOI 10.1007/s00254-005-0001-7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karmegam, U., Chidambaram, S., Prasanna, M.V. et al. A study on the mixing proportion in groundwater samples by using Piper diagram and Phreeqc model. Chin. J. Geochem. 30, 490–495 (2011). https://doi.org/10.1007/s11631-011-0533-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-011-0533-3