Abstract
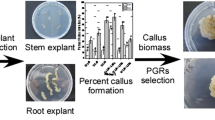
Phlomis armeniaca Willd. is a medicinal plant in the Lamiaceae family endemic to Turkey. The present study describes efficient plant regeneration and callus induction protocols for P. armeniaca and compares phenolic profiles, total phenol and flavonoid contents, and free radical scavenging activity of in vitro-derived tissues. Stem node explants from germinated seedlings were cultured on Murashige and Skoog medium (MS) supplemented with 75 plant growth regulator (PGR) combinations. The highest shoot number per explant, frequency of shoot proliferation, and frequency of highly proliferated, green, compact callus were obtained on MS medium containing 0.25 mg L−1 thidiazuron (TDZ) and 0.25 mg L−1 indole-3-acetic acid (IAA). The best root formation was on MS basal medium (control). Methanol extract of leaves obtained from regenerants contained higher total phenol and flavonoid contents than the callus extract. The callus extract showed stronger free radical scavenging activity than leaves with IC50 [concentration inhibiting 50% of 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical] values of 4.30 ± 0.08 and 2.21 ± 0.04 mg g−1 dry weight in leaves and callus, respectively. Apigenin, caffeic acid, p-coumaric acid, luteolin, rutin hydrate, vanillic acid, ferulic acid, salicylic acid, sinapic acid, and chlorogenic acid were detected by liquid chromatography–electrospray ionization multistage tandem mass spectrometry (LC-ESI-MS/MS) analysis in in vitro-grown leaves and callus tissue. Rutin hydrate, p-coumaric acid, and vanillic acid were found at approximately tenfold higher levels in callus than in leaves. This new micropropagation protocol, the first for P. armeniaca, could be used in industrial production for new herbal tea and germplasm conservation.



Similar content being viewed by others
References
Babbar N, Oberoi HS, Uppal DS, Patil RT (2011) Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Intern 44:391–396
Baytop T (1999) Therapy with medicinal plants in Turkey; today and in future (in Turkish). Istanbul University Press, Istanbul
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT––Food Sci Technol 28:25–30
Calis I, Kirmizibekmez H, Ersoz T, Saracoglu I, Donmez Ali A, Mitova M, Handjieva N, Popov S (2002) Iridoid, phenylethanoid flavonoid glycosides from Phlomis sintenisii. Turk Acta Pharm Turc 44:195–203
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Cook NC, Samman S (1996) Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. Nutr Biochem 7:66–76
Dalar A, Konczak I (2014) Cichorium intybus from eastern Anatolia: phenolic composition, antioxidant and enzyme inhibitory activities. Ind Crop Prod 60:79–85
De Gyves EM, Sparks CA, Fieldsend AF, Lazzeri PA, Jones HD (2001) High frequency of adventitious shoot regeneration from commercial cultivars of evening primrose (Oenothera spp.) using thidiazuron. Ann Appl Biol 138:329–332
Demirci F, Guven K, Demirci B, Dadandi MY, Baser KHC (2008) Antibacterial activity of two Phlomis essential oils against food pathogens. Food Control 19:1159–1164
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agr Food Chem 50:3010–3014
Ersoz T, Ivancheva S, Akbay P, Sticher O, Calis I (2001) Iridoid and phenylethanoid glycosides from Phlomis tuberosa L. Z Naturforsch C 56:695–698
Fernandes de Oliveira AM, Sousa Pinheiro L, Souto Pereira CK, Neves Matias W, Albuquerque Gomes R, Souza Chaves O, Vanderlei de Souza MF, Nóbrega de Almeida R, Simões de Assis T (2012) Total phenolic content and antioxidant activity of some Malvaceae family species. Antioxidants 1:33–43
Gençay A (2007) Cizre (Şırnak)' nin etnobotanik özellikleri. Yüzüncü Yıl Üniversitesi, Fen Bilimleri Enstitüsü, Yüksek Lisans Tezi, Van, Turkey
Gonçalves S, Romano A (2013) In vitro culture of lavenders (Lavandula spp.) and the production of secondary metabolites. Biotechnol Adv 31:166–174
Grzegorczyk-Karolak I, Kuzma L, Wysokinska H (2015) The effect of cytokinins on shoot proliferation, secondary metabolite production and antioxidant potential in shoot cultures of Scutellaria alpina. Plant Cell Tissue Organ Cult 122:699–708
Gülçin I, Oktay M, Kireçci E, Küfrevioğlu İÖ (2003) Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem 83:371–382
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Kabouche A, Kabouche Z, Seguin E, Tillequin F, Bruneau C (2005) A phenylethanoid glycoside and flavonoids from Phlomis crinita (Cav.) Lamiaceae. Biochem Syst Ecol 33:813–816
Karakas FP, Turker AU (2013) An efficient in vitro regeneration system for Bellis perennis L. and comparison of phenolic contents of field-grown and in vitro-grown leaves by LC-MS/MS. Ind Crop Prod 48:162–170
Karakas FP, Yildirim AB, Bayram R, Yavuz ZP, Gepdiremen A, Turker AU (2015) Antiproliferative activity of some medicinal plants on human breast and hepatocellular carcinoma cell lines and their phenolic contents. Trop J Pharm Res 14:1787–1795
Limem-Ben Amor I, Boubaker J, Ben Sgaier M, Skandrani I, Bhouri W, Neffati A, Kilani S, Bouhlel I, Ghedira K, Chekir-Ghedira L (2009) Phytochemistry and biological activities of Phlomis species. J Ethnopharmacol 125:183–202
Mahendran G, Narmatha Bai V (2014) Micropropagation, antioxidant properties and phytochemical assessment of Swertia corymbosa (Griseb.) Wight ex C. B. Clarke: a medicinal plant. Acta Physiol Plant 36:589–603
Mallon R, Rodriguez-Oubina J, Gonzalez ML (2011) Shoot regeneration from in vitro-derived leaf and root explants of Centaurea ultreiae. Plant Cell Tissue Organ Cult 106:523–530
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin–cytokinin-regulated development. Proc Natl Acad Sci USA 101:8039–8044
Pehlivan Karakas F, Sahin G, Turker A (2016) Enhancement of direct shoot regeneration and determination of bioactive secondary metabolites in leaves of Galega officinalis L. Turk J Biol (in press). doi:10.3906/biy-1603-70
Pokorny J (1991) Natural antioxidants for food use. Trends Food Sci Technol 2:223–227
Saracoglu I, Inome M, Calis I, Ogihara Y (1995) Studies on constituents with cytotoxic and cytostatic activity of 2 Turkish medicinal-plants Phlomis armeniaca and Scutellaria salviifolia. Biol Pharm Bull 18:1396–1400
Sarikurkcu C, Uren MC, Tepe B, Cengiz M, Kocak MS (2015) Phlomis armeniaca: phenolic compounds, enzyme inhibitory and antioxidant activities. Ind Crop Prod 78:95–101
Shahidi F (2000) Antioxidants in food and food antioxidants. Nahrung 44:158–163
Turker AU, Yıldırım AB (2013) Evaluation of antibacterial and antitumor activities of some Turkish endemic plants. Trop J Pharm Res 12:1003–1010
Turker A, Yucesan B, Gurel E (2009) In vitro regeneration of Achillea millefolium L. from shoot-tips and root segments of seedlings. J Plant Biochem Biotechnol 18:61–69
Uysal A, Gunes E, Sarikurkcu C, Celik H, Durak Y, Uren MC (2016) New prospective materials for chemoprevention: three Phlomis. Br J Pharm Res 10(3):1–13
Verpoorte R, Memelink J (2002) Engineering secondary metabolite production in plants. Curr Opin Biotechnol 13:181–187
Yalcin FN, Ersoz T, Akbay P, Calis I, Donmez AA, Sticher O (2003) Iridoid and phenylpropanoid glycosides from Phlomis samia, P. monocephala and P. carica. Turk J Chem 27:295–305
Yasar S, Fakir H, Erbas S (2010) Gas chromatographic (GC-GC/MS) analysis of essential oil of Phlomis armeniaca Willd. from mediterranean region in Turkey. Asian J Chem 22:2887–2890
Yucesan B, Turker AU, Gurel E (2007) TDZ-induced high frequency plant regeneration through multiple shoot formation in witloof chicory (Cichorium intybus L.). Plant Cell Tissue Organ Cult 91:243–250
Yumrutas O, Saygıdeger SD (2012) Determination of antioxidant and antimutagenic activities of Phlomis armeniaca and Mentha pulegium. J App Pharm Sci 2:36–40
Acknowledgement
This study was supported by grants from the Abant Izzet Baysal University Research Foundation (project no. 2013.03.01.625).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Rakhi Chaturvedi
Rights and permissions
About this article
Cite this article
Karakas, F.P., Turker, A.U. Improvement of shoot proliferation and comparison of secondary metabolites in shoot and callus cultures of Phlomis armeniaca by LC-ESI-MS/MS analysis. In Vitro Cell.Dev.Biol.-Plant 52, 608–618 (2016). https://doi.org/10.1007/s11627-016-9792-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-016-9792-3