Abstract
A mixture component design was applied to testing the Murashige and Skoog (MS) medium nitrogen salts (NH4NO3 and KNO3) in order to optimize growth medium nitrogen for shoot cultures of red raspberries and a range of Rubus germplasm. All other components were those of MS medium with 2.5× MS mesos (CaCl2, MgSO4, KH2PO4) nutrients developed in an earlier study. Eight red raspberry (Rubus idaeus L.) cultivars and one accession of Rubus odoratus L. were evaluated for overall quality, shoot length, proliferation, leaf characteristics, and physiological disorders after 9-wk growth on each treatment. Twelve Rubus genotypes were later tested with the five best treatments. The concentration of NO3 − in the medium was the most significant factor for improved shoot quality for most cultivars. The effects of the two-component mixture (NH4 + and K+) and interactions between the mixture components and NO3 − were found in many growth responses. Combinations of intermediate or high NO3 − (40–60 mM), and intermediate to high NH4 + (30–45 mM) produced the best growth in most cultivars. The 2.5× mesos MS medium with standard MS nitrogen (20 mM NH4 + and 40 mM NO3 −) produced significantly better growth for most genotypes than unmodified MS. Three other nitrogen treatments also improved shoot quality for many genotypes: lower NH4 + with lower total nitrogen (10 mM NH4 + and 40 mM NO3 −) or intermediate or high concentrations of both nitrogen components and higher total nitrogen (30 mM NH4 + and 60 mM NO3 −; 45 mM NH4 + and 60 mM NO3 −) than that of MS medium.





Similar content being viewed by others
References
Anderson WC (1980) Tissue culture propagation of red and black raspberries, Rubus idaeus and R. occidentalis. Acta Hortic 112:13–20
Cousson A, Van Tran Thanh K (1993) Influence of ionic composition of the culture medium on de novo flower formation in tobacco thin cell layers. Can J Bot 71:506–511
Design-Expert (2010) Stat-Ease, Inc., Minneapolis, MN
Evans N (1993) A preliminary study on the effects of nitrogen supply on the growth in vitro of nine potato genotypes (Solanum spp). J Exp Bot 44:837–841
Leljak-Levanić D, Bauer N, Mihaljević S, Jelaska S (2004) Somatic embryogenesis in pumpkin (Cucurbita pepo L.): control of somatic embryo development by nitrogen compounds. J Plant Physiol 161:229–236
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Menke-Milczarek I, Zimny J (2001) NH4 + and NO3 − requirement for wheat somatic embryogenesis. Acta Physiol Plant 23:37–42
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Niedz RP, Evens TJ (2006) A solution to the problem of ion confounding in experimental biology. Nat Methods 3:417
Niedz RP, Evens TJ (2007) Regulating plant tissue growth by mineral nutrition. In Vitro Cell Dev Biol Plant 43:370–381
Niedz RP, Evens TJ (2008) The effects of nitrogen and potassium nutrition on the growth of nonembryogenic and embryogenic tissue of sweet orange (Citrus sinensis (L.) Osbeck). BMC Plant Biol 8:126
Niedz RP, Hyndman SE, Evens TJ, Weathersbee AA III (2014) Mineral nutrition and in vitro growth of Gerbera hybrida (Asteraceae). In Vitro Cell Dev Biol Plant 50:458–470
Nowak B, Miczyński K, Hudy L (2007) The effect of total inorganic nitrogen and the balance between its ionic forms on adventitious bud formation and callus growth of ‘Węgierka Zwykła’ plum (Prunus domestica L.). Acta Physiol Plant 29:479–484
Poothong S, Reed BM (2014) Modeling the effects of mineral nutrition for improving growth and development of micropropagated red raspberries. Sci Hortic 165:132–141
Preece J (1995) Can nutrient salts partially substitute for plant growth regulators? Plant Tissue Cult Biotechnol 1:26–37
Ramage CM, Williams RR (2002) Inorganic nitrogen requirements during shoot organogenesis in tobacco leaf discs. J Exp Bot 53:1437–1443
Reed BM, Wada S, DeNoma J, Niedz RP (2013) Mineral nutrition influences physiological responses of pear in vitro. In Vitro Cell Dev Biol Plant 49:699–709
Scherer HW, Mackown CT, Leggett JE (1984) Potassium-ammonium uptake interactions in tobacco seedlings. J Exp Bot 35:1060–1070
Wada S, Niedz RP, Reed BM (2015) Determining nitrate and ammonium requirements for optimal in vitro response of diverse pear species. In Vitro Cell Dev Biol Plant 51:19–27
Zou C, Shen J, Zhang F, Guo S, Rengel Z, Tang C (2001) Impact of nitrogen form on iron uptake and distribution in maize seedlings in solution culture. Plant Soil 235:143–149
Zou C, Wang X, Wang Z, Zhang F (2005) Potassium and nitrogen distribution pattern and growth of flue-cured tobacco seedlings influenced by nitrogen form and calcium carbonate in hydroponic culture. J Plant Nutr 28:2145–2157
Acknowledgments
Funding for this study was from the US Department of Agriculture, Agricultural Research Service CRIS project 5358-21000-033D. Sukalya Poothong acknowledges the scholarship from the Ministry of Science and Technology (Thailand) for her Ph.D. studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: David Duncan
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement 1
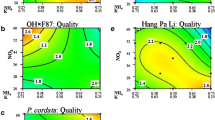
Overall quality graphs (rated 1=poor [dark blue] to 3=good [red]) showing the growth appearance on standard MS medium compared to three levels of NO3 − in raspberry shoots of ‘Canby’ (a), ‘Chilliwack’ (b), ‘Indian Summer’ (c), ‘Malling Exploit’ (d), ‘Scepter’ (e), ‘Trailblazer’ (f), ‘Tulameen’ (g), ‘Willamette’ (h), and R. odoratus (i) (PPTX 8113 kb)
Supplement 2
The effects of ammonium and potassium proportion (NH4 +:K+) and total NO3 − on the number of shoots, leaf color (SPAD reading), and leaf area (cm2) in additional raspberry cultivars. (PPTX 555 kb)
Supplement 3
The effects of the two mixture components (NH4 + and K+) and the numeric factor (NO3 −) on physiological disorders in nine raspberry cultivars grown on 2.5× MS mesos. (DOCX 29 kb)
Supplement 4
The effects of ammonium and potassium proportion (NH4 +:K+) and total NO3 − on leaf spot elimination (rated 1=presence of leaf spots to 3=absent), callus elimination (1=callus ≥5 mm to 3=absent), and hyperhydricity elimination (1=presence of hyperhydricity to 3=absent) for red raspberry shoot cultures. (PPTX 429 kb)
Supplement 5
Best treatments (ammonium and potassium proportions (NH4 +:K+) and total NO3 −) based on the general linear model (GLM) in nine raspberry cultivars. (DOCX 30 kb)
Supplement 6
The effects of the best nitrogen treatments on overall quality and plant appearance of 12 Rubus cultivars and species when analyzed with or without shoots grown on the standard MS medium. (DOCX 34 kb)
Supplement 7
The effects of modified nitrogen levels with 2.5× MS mesos medium on leaf color (SPAD) (a), leaf area (cm2) (b), leaf spot elimination (1=leaf spots present on many leaves, 2=less than 2 leaves, 3=absence) (c), and callus elimination (1=callus ≥5 mm, 2=callus <5 mm, and 3=absence) (d). The mixture component is listed first followed by the total N (mM) and the treatment (e.g., Trt. 3) based on Table 1. Vertical bars indicate standard errors. Means followed by the same letter are not significantly different (p<0.05). Red points represent the responses of shoots grown on standard MS (Trt. 10) but not included in statistical analysis. (PPTX 355 kb)
Rights and permissions
About this article
Cite this article
Poothong, S., Reed, B.M. Optimizing shoot culture media for Rubus germplasm: the effects of NH4 +, NO3 −, and total nitrogen. In Vitro Cell.Dev.Biol.-Plant 52, 265–275 (2016). https://doi.org/10.1007/s11627-016-9750-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-016-9750-0