Abstract
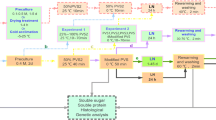
In a bid to better conserve endangered terrestrial orchids, we detail cryogenic research using a widely distributed terrestrial orchid, Caladenia latifolia, as a model species for development of cryopreservation for primary (seed generated) and secondary (adventitious) protocorms. Primary protocorm cryopreservation (using droplet vitrification) involved a number of experimental lines of inquiry: investigation of a suitable plant vitrification solution (PVS) by comparing three variants of a standard PVS (2, 3 and 4), determining the most suitable primary protocorm developmental stage for successful cryopreservation, testing the effectiveness of a preculture medium treatment prior to cryopreservation, and investigating temperature preconditioning at the preculture stage as well as different components of the recovery medium. Primary protocorms were generated using asymbiotic in vitro germination media developed by the authors specifically for the test species (half-strength MS macroelements and microelements with 5% (v/v) fresh filter sterilized coconut water). Secondary protocorms were propagated using an in vitro proliferation medium (½ MS with 5 μM α-naphthaleneacetic acid + 2 μM 6-benzylaminopurine). A modified preconditioning step was developed, involving incubation on ½ MS with 0.2 M raffinose for 48 h at 15°C instead of 20°C. The standard recovery medium (½ MS 1 μM zeatin + 0.5 μM gibberellic acid) was replaced after the first week following warming from liquid nitrogen (LN), with asymbiotic germination medium (½ MS + 5% (v/v) coconut water) for the remainder of the recovery phase. This new step increased the survival of primary protocorms from 68 to 85%. The average post-cryostorage regeneration of plants from primary protocorms increased from 17 to 48% after a 6-wk incubation. A similar protocol increased the survival of secondary protocorms from 63 to 84%. Regeneration of plants from secondary cryostored protocorms increased from 11 to 26% after 14 wk. The protocols developed here provide a useful template for advancing cryoconservation of other orchid taxa, particularly rare and threatened species.



Similar content being viewed by others
References
Antony JJJ, Sinniah UR, Keng CL, Pobathy R, Khoddamzadeh AA, Subramaniam S (2011) Selected potential encapsulation-dehydration parameters on Dendrobium Bobby Messina protocorm-like bodies using TTC analysis. Aust J Crop Sci 5:1817–1822
Ashmore S, Azimi M, Drew RA (2001) Cryopreservation trials in Carica papaya. Acta Hort 560:117–120
Barraco G, Sylvestre I, Engelmann F (2011) Comparing encapsulation-dehydration and droplet-vitrification for cryopreservation of sugarcane (Saccharum spp.) soot tips. Sci Hort 130:320–324
Batty AL, Dixon KW, Brundrett M, Sivasithamparam K (2001) Constraints to symbiotic germination of terrestrial orchid seed in Mediterranean bushland. New Phytol 152:511–520
Batty AL, Brundrett MC, Dixon KW, Sivasithamparam K (2006) New methods to improve symbiotic propagation of terrestrial orchid seedlings from axenic culture to soil. Aust J Bot 54:367–374
Brundrett MC (2007) Scientific approaches to Australian temperate terrestrial orchid conservation. Aust J Bot 55:293–307
Bustam BM, Dixon KW, Bunn E (2014a) In vitro propagation of temperate Australian terrestrial orchids: revisiting asymbiotic compared to symbiotic germination. Bot J Linn Soc 176(4):556–566. doi:10.1111/boj.12216
Bustam BM, Dixon KW, Bunn E (2014b) Proliferation and harvesting of secondary protocorms as a novel means for improving propagation of terrestrial orchids. Aust J Bot 62(7):614–621. doi:10.1071/BT14291
Chang Y, Reed BM (2000) Extended alternating-temperature cold acclimation and culture duration improve pear shoot cryopreservation. Cryobiology 40:311–322
Clements MA, Muir H, Cribb PJ (1986) A preliminary report on the symbiotic germination of European terrestrial orchids. Kew Bull 41:437–445
Coates DJ, Dixon KW (2007) Current perspectives in plant conservation biology. Aust J Bot 55:187–193
Ellis D, Skogerboe D, Andre C, Helier B, Volk G (2006) Implementation of garlic cryopreservation techniques on the national plant germplasm system. Cryog Lett 27(2):99–106
Flachsland E, Terada G, Socchi A, Rey H, Mroginski L, Engelmann F (2006) Cryopreservation of seeds and in-vitro cultured protocorms of Oncidium bifolium Sims. (Orchidaceae) by encapsulation-dehydration. Cryog Lett 27:235–242
Gogoi K, Kumaria S, Tandon P (2013) Cryopreservation of Dendrobium eburneum Lindl. And C. hookerianum Rchb.f., two threatened and vulnerable orchids via encapsulation-dehydration. In Vitro Cell Dev Biol Plant 49:248–254
Hay FR, Merritt DJ, Soanes JA, Dixon KW (2010) Comparative longevity of Australian orchid (Orchidaceae) seeds under experimental and low temperature storage conditions. Bot J Linn Soc 164:26–41
Heringer AS, Steinmacher DA, Fraga HPF, Veira LN, Ree JF, Guerra MP (2013) Global DNA methylation profiles of somatic embryos of peach palm (Bactris gasipaes Kunth) are influenced by cryoprotectants and droplet-vitrification cryopreservation. Plant Cell Tiss Organ Cult 114:365–372
Hirano T, Godo T, Mii M, Ishikawa K (2005) Cryopreservation of immature seeds of Bletilla striata by vitrification. Plant Cell Rep 23:534–539
Hooi TH, James J, Julkiflee A, Poobaty R, Gnasekaram P, Subramaniam S (2010) A novel approach for preliminary PVS2 vitrification optimization parameters of Dendrobium sonia-28 orchid with evan blue staining. Adv Environ Biol 4(2):284–290
Institute for statistics and Mathematics of WirtshaftuniversitatWein (2014) www.r-project.org
Ishikawa K, Harata K, Mii M, Sakai A, Yoshimatsu K, Shimomura K (1997) Cryopreservation of zygotic embryos of a Japanese terrestrial orchid (Bletilla striata) by vitrification. Plant Cell Rep 16:754–757
Jitsopakul N, Thammasiri K, Ishikawa K (2008) Cryopreservation of Vanda coerulea protocorms by encapsulation-dehydration. Cryog Lett 29:253–260
Jusatitis M, Sorensen B (1993) Germination of Pterostylis arenicola—an endangered greenhood from South Australia. Orchadian 11:18–22
Kaczmarczyk A, Funnekotter B, Menon A, Phang PY, Al-Hanbali A, Bunn E, Mancera RL (2012) Current issues in plant cryopreservation. In: Katkov II (ed) Current frontiers in cryobiology. InTechOpen, p 417–437
Kaczmarczyk A, Turner SH, Bunn E, Manceera RL, Dixon KW (2013) Development of cryopreservation for Loxocaryacinerea—an endemic Australian plant species important forpost-mining restoration. Cryog Lett 34:508–519
Khoddamzadeh AA, Sinniah UR, Lynch P, Kadir AA, Kadzimin SB, Mahmood M (2011) Cryopreservation of protocorm-like bodies (PLBs) of Phalaenopsis bellina (Rchb.f) Christenson by encapsulation-dehydration. Plant Cell Tiss Organ Cult 107(3):471–481
Kim HH, Lee SC (2012) ‘Personalisation’ of droplet-vitrification protocols for plant cells: a systematic approach to optimising chemical and osmotic effects. Cryog Lett 33(4):271–279
Kim HH, Lee YG, Shin DJ, KO HC, Gwag JG, Cho EG, Engelmann F (2009) Development of alternative plant vitrification solutions in droplet-vitrification procedures. Cryog Lett 30(5):320–334
Lee YI, Hsu ST, Yeung C (2013) Orchid protocorm-like bodies are somatic embryos. Am J Bot 100(11):2121–2131
Liu H, Feng CL, Luo YB, Chen BS, Wang ZS, Gu HY (2010) Potential challenges of climate change to orchid conservation in a wild orchid hotspot in southwestern China. Bot Rev 76:174–192
Maneerattanarungjoy P, Bunnang S, Monthatong M (2007) In vitro conservation of Cleistoma areitinum (Rchb.f.) Garay, rare Thai orchid species by an encapsulation-dehydration method. Asian J Plant Sci 6:1235–1240
McCarty JP (2001) Ecological consequences of recent climate change. Conserv Biol 15:320–331
Merritt DJ, Hay FR, Swarts ND, Sommerville KD, Dixon KW (2014) Ex situ conservation of orchid germplasm. Int J Plant Sci 175:46–58
Mohanty P, Das M, Kumaria S, Tandon P (2012) High-efficiency cryopreservation of the medicinal orchid Dendrobium nobile Lindl. Plant Cell Tiss Organ Cult 109:297–305
Nicholls N (2004) The changing nature of Australian droughts. Climate Change 63:1473–1480
Nikishina TV, Popova EV, Vakhrameeva MG, Varlygina TI, Kolomeitseva GL, Burov AV, Popovich EA, Shirokov AI, Shumilov VY, Popov AS (2007) Cryopreservation of seeds and protocorm of rare temperate orchids. Fiziolog Rastenii 54:137–143
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus-officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91(1):67–73
Nurfadillah S (2010) Conservation of critically endangered orchid Drakaea elastica Lindl. in the context of nutritional requirements of saprophytic competency of the mycorrhizal fungus and its propagation. MSc Thesis. The University of Western Australia, Perth
Phillips RD (2010) Landscape, pollinator and mycorrhizal specificity and their contribution to rarity in Drakaea (hammer orchid). PhD Thesis. The University of Western Australia, Perth
Popova EV, Nikishina TV, Kolomeitseva GL, Popov AS (2003) The effect of seed cryopreservation on the development of protocorms by the hybrid orchid Bratonia. Russ J Plant Physiol 50:750–755
Pornchuti W, Thammasiri K (2008) Cryopreservation of protocorms of Dendrobium cariniferum RCHB.F. Acta Hort 788:63–68
Ramsey RR, Dixon KW, Sivasithamparam K (1986) Patterns of infection and endophytes associated with Western Australian orchids. Lindleyana 1:203–214
Reed BM, Okut N, D’Achino J, Narver L, DeNoma J (2003) Cold storage and cryopreservation of hops (Humulus L.) shoot cultures through application standard protocol. Cryog Lett 24:389–396
Rong HS, Hua YM (2012) High-efficiency encapsulation-vitrification protocols for cryopreservation of embryonic calli of the oriental medicinal plant Anemarrhena asphodeloides BUNGE. Cryog Lett 33:190–200
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Reports 9:30–33
Senula A, Keller ERJ, Sanduijav T, Yohannes T (2007) Cryopreservation of cold-acclimated mint (Mentha spp.) shoot tips using a simple vitrification protocol. Cryog Lett 28:1–12
Sinniah UM, Gantait S (2013) Cryopreservation of immature Parkiosa speciosa Hassk. Zygotic embryogenic axes following desiccation or exposure to vitrification solution. Acta Physiol Plant 35:629–2634
Sommerville KD, Siemon JP, Wood CB, Offord CA (2008) Simultaneous encapsulation of seed and mycorrhizal fungi for long term storage and propagation of terrestrial orchids. Aust J Bot 56:609–615
Swarts ND, Dixon KW (2009) Terrestrial orchid conservation in the age of extinction. Ann Bot 104:543–556
Thuiller W, Richardson DM, Pysek P, Midgley GF, Hughes GO, Rouget M (2005) Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob Change Biol 11:2234–2250
Towill LE (1990) Cryopreservation of isolated mint shoot tips by vitrification. Plant Cell Rep 9:178–180
Towill LE, Bonnart R, Volk GM (2006) Cryopreservation of Arabidopsis thaliana shoot tips. Cryog Lett 27:353–360
Urugami A, Sakai A, Nagai M, Takahashi T (1989) Survival of cultured-cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification. Plant Cell Rep 8:418–421
Watanawikkit P, Tantiwiwat S, Bunn E, Dixon KW, Chayanarit K (2012) Cryopreservation of in vitro-propagated protocorms of Caladenia for terrestrial orchid conservation. Bot J Linn Soc 170:277–282
Wright M, Cross R, Dixon K, Huynh T, Lawrie A, Nesbitt L, Pritchard A, Swarts N, Thomson R (2009) Propagation and reintroduction of Caladenia. Aust J Bot 57:373–387
Yin M, Hong S (2009) Cryopreservation of Dendrobium candidum Wall. ex Lindl. Protocorm like bodies by encapsulation-vitrification. Plant Cell Tiss Organ Cult 98:179–185
Yin LL, Poobathy R, James J, Julkifle AL, Subramaniam S (2011) Preliminary investigation of cryopreservation by encapsulation-dehydration technique on Brassidium shooting star orchid hybrid. African J Biotech 10:4665–4672
Yin ZT, Bi WL, Chen L, Zhao B, Volk GM, Wang QC (2014) An efficient, widely applicable cryopreservation of Lilium shoot tips by droplet vitrification. Acta Physiol Plant 36:1683–1692
Zettler LW (1997) Terrestrial orchid conservation by symbiotic seed germination: techniques and perspectives. Selbyana 18:188–194
Acknowledgments
We thank the Indonesian Directorate General of Higher Education for providing the first author with a PhD scholarship. We also thank our colleagues at Kings Park and Botanic Garden for valuable discussions concerning experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
Rights and permissions
About this article
Cite this article
Bustam, B.M., Dixon, K. & Bunn, E. A cryopreservation protocol for ex situ conservation of terrestrial orchids using asymbiotic primary and secondary (adventitious) protocorms. In Vitro Cell.Dev.Biol.-Plant 52, 185–195 (2016). https://doi.org/10.1007/s11627-015-9732-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9732-7