Abstract
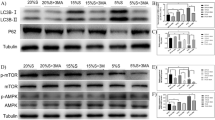
The current study was conducted to evaluate the functions of μ-calpain (CAPN1), calpastatin, HSPs (heat shock proteins), and caspases during myogenesis and cell death induced by sodium azide (NaN3) hypoxia. The cell samples were divided into three groups: satellite cells formed at confluent monolayer (stage 1), stage 1 cells fusion into myotubes on d eight post-differentiation (stage 2), and stage 2 cells treated with 1 mM NaN3 for 24 h (stage 3). Real-time RT-PCR showed that stage 2 cells had increased CAPN1, calpastatin, caspase 7, and CARD9 (Caspase activation and recruitment domain 9) mRNA expressions compared to stage 1 cells (*p < 0.05). By Western blotting caspase 3, caspase 7, caspase 8, and caspase 9 protein levels increased in cells at stage 2 compared to cells at stage 1 (*p < 0.05). Real-time RT-PCR showed that stage 3 cells had increased CAPN1, calpastatin, caspase 7, HSP70 (70 kDA heat shock proteins), and HSP90 (90 kDA heat shock proteins-alpha) and decreased CARD9 mRNA expression compared to stage 2 cells (*p < 0.05). Stage 3 samples had increase caspase 7 and caspase 12 activities compared to stage 2 samples, and by Western blotting protein levels of both HSP70 and HSP90 expressions, increased significantly under hypoxia condition (*p < 0.05). Here, we conclude that CAPN1, calpastatin, caspase 3, caspase 7, caspase 8, and CARD9 have important roles for satellite cell myogenesis; and that caspase 7, 12, HSP70, and HSP90 are involved in the process of apoptotic cell death under hypoxia conditions and we speculate that these proteins may be involved in early postmortem proteolysis and meat tenderization.





Similar content being viewed by others
References
Ahn J. H.; Ko Y. G.; Park W. Y.; Kang Y. S.; Chung H. Y.; Seo J. S. Suppression of ceramide-mediated apoptosis by HSP70. Mol. Cells 9: 200–206; 1999.
Allen R. E. Muscle cell culture as a tool in animal growth research. Fed. Proc. 46: 290–294; 1987.
Altschuld R. A.; Hostelter J. R.; Brierley G. P. Response of isolated rat heart cells to hypoxia, reoxygenation and acidosis. Circ. Res. 49: 307–316; 1981.
Balcerzak D.; Cottin P.; Poussard S.; Cucuron A.; Brustis J. J.; Ducastaing A. Calpastatin-modulation of m-calpain activity is required for myoblast fusion. Eur. J. Cell Biol. 75: 247–253; 1998.
Barnoy S.; Glaser T.; Kosower N. S. The role of calpastatin (the specific calpain inhibitor) in myoblast differentiation and fusion. Biochem. Biophys. Res. Commun. 220: 933–938; 1996.
Barnoy S.; Supino-Rosin L.; Kosower N. S. Regulation of calpain and calpastatin in differentiating myoblasts: mRNA levels, protein synthesis and stability. Biochem. J. 351: 413–420; 2000.
Beere H. M. The stress of dying: the role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 117: 2641–2651; 2000.
Belizario J. E.; Lorite M. J.; Tisdale M. J. Cleavage of caspases-1, -3, -6, -8 and −9 substrates by proteases in skeletal muscles from mice undergoing cancer cachexia. Br. J. Cancer 84: 1135–1140; 2001.
Bertin J. Y.; Guo L.; Wang S. M.; Srinivasula M. D.; Jacobson J. L.; Poyet S.; Merriam M. Q.; Du M. J.; Dyer K. E.; Robison P. S.; DiStefano A. E. S. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappaB. J. Biol. Chem. 275: 41082–41086; 2000.
Bischoff R. Enzymatic liberation of myogenic cells from adult rat muscle. Anat. Rec. 180: 645–662; 1974.
Brustis J. J.; Elamrani N.; Balcerzak D.; Safwate A.; Soriano M.; Poussard S.; Cottin P.; Ducastaing A. Rat myoblast fusion requires exteriorized m-calpain activity. Eur. J. Cell Biol. 64(2): 320–327; 1994.
Cassar-Malek I.; Langloisa N.; Picarda B.; Geaya Y. Regulation of bovine satellite cell proliferation and differentiation by insulin and triiodothyronine. Domest. Anim. Endocrinol. 17: 373–388; 1999.
Chen S. J.; Bradley M. E.; Lee T. C. Chemical hypoxia triggers apoptosis of cultured neonatal rat cardiac myocytes: modulation by calcium-regulated proteases and protein kinases. Mol. Cell Biochem. 178: 141–149; 1998.
Concannon C. G.; Gorman A. M.; Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis 8: 61–70; 2003.
Cottin P.; Brustis J. J.; Poussard S.; Elamrani N.; Broncard S.; Ducastaing A. Ca2+-dependent proteinases (calpains) and muscle cell differentiation. Biochim. Biophys. Acta 1223: 170–178; 1994.
Creagh E. M.; Carmody R. J.; Cotter T. G. Heat shock protein 70 inhibits caspase-dependent and -independent apoptosis in Jurkat T cells. Exp. Cell Res. 257: 58–66; 2000.
Dodson M. V.; Martin E. L.; Brannon M. A.; Mathison B. A.; Mcfarland D. C. Optimization of bovine satellite cell derived myotube formation in vitro. Tissue Cell 19: 159–166; 1987.
Dodson M. V.; McFarland D. C.; Martin E. L.; Brannon M. A. Isolation of satellite cells from ovine skeletal muscle. J. Tissue Cult. Methods 10: 233–237; 1986.
Earnshaw W. C.; Martins L. M.; Kaufmann S. H. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68: 383–424; 1999.
Ebisui C.; Tsujinaka T.; Kido Y.; Iijima S.; Yano M.; Shibata H.; Tanaka T.; Mori T. Role of intracellular proteases in differentiation of L6 myoblast cells. Biochem. Mol. Biol. Int. 32: 515–521; 1994.
Fernando P.; Kelly J. F.; Balazsi K.; Slack R. S.; Megeney L. A. Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. U.S.A. 99: 11025–11030; 2002.
Fidzianska A.; Kaminska A.; Glinka Z. Muscle cell death. Ultrastructural difference between muscle cell necrosis and apoptosis. Neuropatol. Pol. 29: 19–28; 1991.
Fischer D.; Matten J.; Reimann J.; Bönnemann C.; Schröder R. Expression, localization and functional divergence of αB-crystallin and heat shock protein 27 in core myopathies and neurogenic atrophy. Acta Neuropathol. 101: 297–304; 2002.
Fuentes-Prior P.; Salvesen G. S. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem. J. 384: 201–232; 2004.
Goll D. E.; Thompson V. F.; Li H.; Wei W.; Cong J. The calpain system. Physiol. Rev. 83: 731–801; 2003.
Gustafsson A. B.; Gottlieb R. A. Mechanisms of apoptosis in the heart. J. Clin. Immunol. 23: 447–459; 2003.
Hayashi M.; Inomata M.; Kawashima S. Function of calpains: possible involvement in myoblast fusion. Adv. Exp. Med. Biol. 389: 149–154; 1996.
Hwang I. H. Application of gel-based proteome anaysis techniques to studying pros-mortem proteolysis in meat. Asian-Australasian J. Anim. Sci. 17: 1296–1302; 2004.
Inomata K.; Tanaka H. Protective effect of benidipin against sodium azide-induced cell death in cultured neonatal rat cardiac myocytes. J. Pharmacol. Sci. 93: 163–170; 2003.
Jaattela M.; Wissing D.; Kokholm K.; Kallunki T.; Egeblad M. Hsp70 exerts its anit-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17: 6124–6134; 1998.
Kemp C. M.; Bardsley R. G.; Parr T. Changes in caspase activity during the postmortem conditioning period and its relationship to shear force in porcine longissimus muscle. J. Anim. Sci. 84: 2841–2846; 2006.
Kemp C. M.; King D. A.; Shackelford S. D.; Wheeler T. L.; Koohmaraie M. The caspase proteolytic system in callipyge and normal lambs in longissimus, semimembranosus, and infraspinatus muscles during postmortem storage. J. Anim. Sci. 87: 2943–2951; 2009.
Kemp C. M.; Sensky P. L.; Bardsley R. G.; Buttery P. J.; Parr T. Tenderness-an enzymatic view. Meat Sci. 84(2): 248–256; 2010.
Kook S. H.; Choi K. C.; Son Y. O.; Lee K. Y.; Hwang I. H.; Lee H. J.; Chang J. S.; Choi I. H.; Lee J. C. Satellite cells isolated from adult hanwoo muscle can proliferate and differentiate into myoblasts and adipose-like cells. Mol. Cells 22: 239–245; 2006.
Kositprapa C.; Zhang B.; Berger Jr. S.; Canty J. M.; Lee T. C. Calpain-mediated proteolytic cleavage of troponin I induced by hypoxia or metabolic inhibition in cultured neonatal cardiomyocytes. Mol. Cell. Biochem. 214: 47–55; 2000.
Kressel M.; Groscurth P. Distinction of apoptotic and necrotic cell death by in situ labelling of fragmented DNA. Cell Tissue Res. 278: 549–556; 1994.
Kwak K. B.; Chung S. S.; Kim O. M.; Kang M. S.; Ha D. B.; Chung C. H. Increase in the level of m-calpain correlates with the elevated cleavage of filamin during myogenic differentiation of embryonic muscle cells. Biochim. Biophys. Acta 1175: 243–249; 1993.
Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680; 1970.
Leeuwenburgh C. Role of apoptosis in sarcopenia. J. Gerontol. A-Biol. Sci. Med. Sci. 58: 999–1001; 2003.
Li C. Y.; Lee J. S.; Ko Y. G.; Kim J. I.; Seo J. S. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J. Biol. Chem. 275: 25665–25671; 2000.
Mauro A. Satellite cells of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9: 493–495; 1961.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65: 55–63; 1983.
Mosser D. D.; Caron A. W.; Bourget L.; Denis-Larose C.; Massie B. Role of human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17: 5317–5327; 1997.
Ohata H.; Trollinger D. R.; Lemasters J. J. Changes in shape and viability of cultured adult rabbit cardiac myocytes during ischemia/reperfusion injury. Res. Commun. Mol. Pathol. Pharmacol. 86: 259–271; 1994.
Ouali A.; Herrera-Mendez C. H.; Coulis G.; Becila S.; Boudjellal A.; Aubry L.; Sentandreu M. A. Revisiting the conversion of muscle into meat and the underlying mechanisms. Meat Sci. 74: 44–58; 2006.
Pandey P.; Saleh A.; Nakazawa A.; Kumar S.; Srinivasula S. M.; Kumar R.; Weichselbaum V.; Nalin C.; Alnemri E. S.; Kufe D.; Kharbanda S. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 19: 4310–4322; 2000.
Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45; 2001.
Poussarda S.; Cottin P.; Brustisa J. J.; Talmata S.; Elamrania N.; Ducastainga A. Quantitative measurement of calpain I and II mRNAs in differentiating rat muscle cells using a competitive polymerase chain reaction method. Bull. Soc. Chim. Biol. 75: 885–890; 1993.
Sandri M. Apoptotic signalling in skeletal muscle fibers during atrophy. Curr. Opin. Clin. Nutr. Metab. Care 5: 249–253; 2002.
Sandri M.; Meslemani A. H.; Sandri C.; Schjerling P.; Vissing K.; Andersen J. L.; Rossini K.; Carraro U.; Angelini C. Caspase 3 expression correlates with skeletal muscle apoptosis in Duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment? J. Neuropathol. Exp. Neurol. 60: 302–312; 2001.
Schollmeyer J. Possible role of calpain I and calpain II in differentiating muscle. Exp. Cell Res. 163: 413–422; 1981.
Solary E.; Dubrez-Daloz L. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood 100: 4446–4453; 2002.
Spencer M. J.; Croall D. E.; Tidball J. G. Calpains are activated in necrotic fibers from mdx dystrophic mice. J. Biol. Chem. 270: 10909–10914; 1995.
Spencer M. J.; Tidball J. G. Calpain translocation during muscle fiber necrosis and regeneration in dystrophindeficient mice. Exp. Cell Res. 226: 264–272; 1996.
Takumaa K.; Moria K.; Leeb E.; Enomotob R.; Babac A.; Matsuda T. Heat shock inhibits hydrogen peroxide-induced apoptosis in cultured astrocytes. Brain Res. 946: 232–238; 2002.
Temm-Grove C. J.; Wert D.; Thompson V. F.; Allen R. E.; Goll D. E. Microinjection of calpastatin inhibits fusion in myoblasts. Exp. Cell Res. 247: 293–303; 1999.
Theil P. K.; Sørensen I. L.; Therkildsen M.; Oksbjerg N. Changes in proteolytic enzyme mRNAs relevant for meat quality during myogenesis of primary porcine satellite cells. Meat Sci. 73: 335–343; 2006.
Vaisid T.; Kosower N. S.; Barnoy S. Caspase-1 activity is required for neuronal differentiation of PC12 cells: cross-talk between the caspase and calpain systems. Biochim. Biophys.Acta - Mol. Cell Res. 1743: 223–230; 2005.
Vignon X.; Beaulaton J.; Ouali A. Ultrastructural localization of calcium in post-mortem bovine muscle: a cytochemical and X-ray microanalytical study. Histochem. J. 21: 403–411; 1989.
Voss O. H.; Batra S.; Kollattukudy S. J.; Gonzalez-Mejja M. E.; Smith J. B.; Doseff A. I. Binding of caspase-3 prodomain to heat shock protein 27 regulates monocyte apoptosis by inhibiting caspase-3 proteolytic activation. J. Biol. Chem. 282: 25088–25099; 2007.
Welch W. J. Mammalian stressre sponse: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol. Rev. 72: 1063; 1992.
Wilson K. P.; Black J. A.; Thomson J. A.; Kim E. E.; Griffith J. P.; Navia M. A.; Murcko M. A.; Chambers S. P.; Aldape R. A.; Raybuck S. A. Structure and mechanism of interleukin-1 beta converting enzyme. Nature 370(6487): 270–5; 1994.
Acknowledgments
It should be acknowledged that this work was partly supported by a grant from the Next-Generation BioGreen 21 Program(No. PJ008191) and a research fund for FTA issues (No. PJ907055), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Yang, Y.B., Pandurangan, M. & Hwang, I. Changes in proteolytic enzymes mRNAs and proteins relevant for meat quality during myogenesis and hypoxia of primary bovine satellite cells. In Vitro Cell.Dev.Biol.-Animal 48, 359–368 (2012). https://doi.org/10.1007/s11626-012-9513-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-012-9513-0