Abstract
Background
Compared to the widely adopted 2–4 months of pre-operative therapy for patients with borderline resectable (BR) or locally advanced (LA) pancreatic ductal adenocarcinoma (PDAC), our institution tends to administer a longer duration before considering surgical resection. Using this unique approach, the aim of this study was to determine pre-operative variables associated with survival.
Methods
Records from patients with BR/LA PDAC who underwent attempt at surgical resection from 1992–2014 were reviewed.
Results
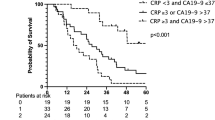
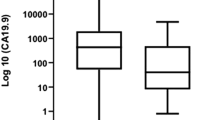
After a median duration of 6 months of pre-operative treatment, 109 patients with BR/LA PDAC (BR 63, LA 46) were explored; 93 (85.3 %) underwent pancreatectomy. Those who received at least 6 months of pre-operative treatment had longer median overall survival (OS) than those who received less (52.8 vs. 32.1 months, P = 0.044). On multivariate analysis, pre-operative treatment duration was the strongest predictor of survival (hazard ratio (HR) 4.79, P = 0.043). However, OS was similar in those whose CA19-9 normalized regardless of whether they received more or less than 6 months of chemotherapy (71.4 vs. 101.8 months, P = 0.930).
Conclusions
Pre-operative CA19-9 decline can guide treatment duration in patients with BR/LA PDAC. We endorse 6 months of therapy except in those patients whose values normalize, where surgery can be considered after a shorter course.




Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29.
Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21.
Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-48.
Malafa MP. Defining borderline resectable pancreatic cancer: emerging consensus for an old challenge. J Natl Compr Canc Netw 2015;13:501-4.
Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33.
Capussotti L, Massucco P, Ribero D, et al. Extended lymphadenectomy and vein resection for pancreatic head cancer: outcomes and implications for therapy. Arch Surg 2003;138:1316-22.
van Geenen RC, ten Kate FJ, de Wit LT, et al. Segmental resection and wedge excision of the portal or superior mesenteric vein during pancreatoduodenectomy. Surgery 2001;129:158-63.
Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 2001;234:758-68.
Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 2006;10:1199-210.
Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol 2009;16:836-47.
Abrams RA, Lowy AM, O’Reilly EM, et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol 2009;16:1751-6.
Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619-27.
Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749-56.
Takahashi H, Ohigashi H, Gotoh K, et al. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg 2013;258:1040-50.
Cho IR, Chung MJ, Bang S, et al. Gemcitabine based neoadjuvant chemoradiotherapy therapy in patients with borderline resectable pancreatic cancer. Pancreatology 2013;13:539-43.
Rose JB, Rocha FG, Alseidi A, et al. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol 2014;21:1530-7.
Takeda Y, Nakamori S, Eguchi H, et al. Neoadjuvant gemcitabine-based accelerated hyperfractionation chemoradiotherapy for patients with borderline resectable pancreatic adenocarcinoma. Jpn J Clin Oncol 2014;44:1172-80.
Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85.
Aristu J, Cañón R, Pardo F, et al. Surgical resection after preoperative chemoradiotherapy benefits selected patients with unresectable pancreatic cancer. Am J Clin Oncol 2003;26:30-6.
Sa Cunha A, Rault A, Laurent C, et al. Surgical resection after radiochemotherapy in patients with unresectable adenocarcinoma of the pancreas. J Am Coll Surg 2005;201:359-65.
Allendorf JD, Lauerman M, Bill A, et al. Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: feasibility, efficacy, and survival. J Gastrointest Surg 2008;12:91-100.
Bickenbach KA, Gonen M, Tang LH, et al. Downstaging in pancreatic cancer: a matched analysis of patients resected following systemic treatment of initially locally unresectable disease. Ann Surg Oncol 2012;19:1663-9.
Strobel O, Berens V, Hinz U, et al. Resection after neoadjuvant therapy for locally advanced, “unresectable” pancreatic cancer. Surgery 2012;152:S33-42.
Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2012;84:1166-71.
Habermehl D, Kessel K, Welzel T, et al. Neoadjuvant chemoradiation with Gemcitabine for locally advanced pancreatic cancer. Radiat Oncol 2012;7:28.
Donahue TR, Isacoff WH, Hines OJ, et al. Downstaging chemotherapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors: influence on patient selection for surgery. Arch Surg 2011;146:836-43.
Kadera BE, Sunjaya DB, Isacoff WH, et al. Locally advanced pancreatic cancer: association between prolonged preoperative treatment and lymph-node negativity and overall survival. JAMA Surg 2014;149:145-53.
Washington K, Berlin J, Branton P, et al. Protocol for the Examination of Specimens From Patients with Carcinoma of the Exocrine Pancreas Version 3.2.0.0. Northfield, IL: College of American Pathologists;2012:1-18.
Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol 2013;2:413-425.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47.
Berger AC, Garcia M Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 2008;26:5918-22.
Tzeng CW, Balachandran A, Ahmad M, et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2014;16:430-8.
Combs SE, Habermehl D, Kessel KA, et al. Prognostic impact of CA 19-9 on outcome after neoadjuvant chemoradiation in patients with locally advanced pancreatic cancer. Ann Surg Oncol 2014;21:2801-7.
Kim TH, Han SS, Park SJ, et al. CA 19-9 level as indicator of early distant metastasis and therapeutic selection in resected pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:743-8.
Yoo T, Lee WJ, Woo SM, et al. Pretreatment carbohydrate antigen 19-9 level indicates tumor response, early distant metastasis, overall survival, and therapeutic selection in localized and unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:623-30.
Iacobuzio-Donahue CA, Fu B, Yachida S, et al.: DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13.
Kannagi R. Carbohydrate antigen sialyl Lewis a--its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J. 2007;30:189-209.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have nothing to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
(DOCX 59 kb)
Supplemental Table 2
(DOCX 128 kb)
Rights and permissions
About this article
Cite this article
Williams, J.L., Kadera, B.E., Nguyen, A.H. et al. CA19-9 Normalization During Pre-operative Treatment Predicts Longer Survival for Patients with Locally Progressed Pancreatic Cancer. J Gastrointest Surg 20, 1331–1342 (2016). https://doi.org/10.1007/s11605-016-3149-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3149-4