Abstract
Introduction
The current standard of care for the management of minimal change chronic pancreatitis (MCCP) is medical management. Controversy exists, however, regarding the use of surgical intervention for MCCP. We hypothesized that total pancreatectomy and islet cell autotransplantation (TPIAT) decreases long-term resource utilization and improves quality of life, justifying initial costs and risks.
Methods
Detailed perioperative outcomes from 46 patients with MCCP populated a Markov model comparing medical management to TPIAT. Mortality, complications, readmission rates, insulin and narcotic use, imaging, and endoscopy were included in the model. Outcomes reported were survival, measured in quality-adjusted life years (QALYs), and costs, in 2013 US dollars.
Results
In medical patients, annual mean hospital admissions were 1.6 (range = 0–11), endoscopy 1.4 (0–6), and imaging (CT/MRI) 1.5 (0–4). In surgical patients, there were no perioperative deaths, with complication and 30-day readmission rates of 47 and 37 %. One year after TPIAT, annual mean admissions, endoscopy, and imaging had decreased to 0.9 (0–4), 0.4 (0–2), and 0.9 (0–5); monthly narcotic use decreased from 138 to 37 morphine equivalents (p = 0.012). Cost and survival for TPIAT versus medical management were $153,575/14.9 QALYs and $196,042/11.5 QALYs, respectively.
Conclusions
In patients with MCCP, TPIAT is associated with decreased cost and increased quality-adjusted survival. Providers and insurers should more enthusiastically embrace TPIAT use as a more effective cost-saving strategy.
Similar content being viewed by others
Introduction
Patients with minimal change chronic pancreatitis (MCCP) may experience debilitating abdominal pain, recurrent acute pancreatitis, and a declining quality of life. Furthermore, the pancreas of a patient with MCCP lacks the traditional morphologic changes characteristic of large duct calcific disease.1 , 2 Consequently, management of these patients is often challenging as they are usually not candidates for traditional surgical procedures or endoscopic management; most patients are managed medically with varying degrees of success.3 The majority of these patients eventually experience a progressive decline in functional status, and while numerous operative strategies have been proposed to address this disease process, each of these series has been limited to small patient populations at single-centers.4 – 10
Our center has been performing total pancreatectomy and islet cell autotransplantation (TPIAT) as a means of treating patients with severe chronic pancreatitis that have failed previous surgical management, and we have also utilized this strategy in patients with genetic and hereditary pancreatitis.11 Many of these latter patients often present with minimal change pancreatitis (MCP), and our experience with these patients has demonstrated a great benefit of TPIAT. Based on this experience, we started applying a similar treatment algorithm to patients that presented with MCCP secondary to other etiologies. TPIAT adequately treats the disease process through complete gland excision while abrogating Type 3c diabetes via relocation of the patient’s own islet cells into the liver. It is also likely that many patients with MCCP have an undetected underlying genetic mutation and may be at higher risk of developing cancer. Thus, the strategy of TPIAT eliminates this risk. While the indications for TPIAT continue to evolve in patients with refractory MCCP, TPIAT is considered as a first-line surgical therapy at a number of institutions, including ours.
Despite the early success, TPIAT remains a substantial undertaking for patients that must carefully consider the associated cost and morbidity, balancing the prospect of long-term insulin and narcotic independence against short-term surgical risks and costs. The aim of this study was to evaluate the cost-effectiveness of TPIAT as a first-line surgical intervention in refractory MCCP using each patient’s preoperative course as the medical management control group. We hypothesize that TPIAT decreases long-term resource utilization and improves quality of life, justifying the initial costs and risks of the procedure.
Materials and Methods
Study Cohort
The University of Cincinnati Pancreatic Disease Center TPIAT Clinical Database was queried to identify all patients undergoing TPIAT for MCCP from 1999 to 2013 (n = 84). Pertinent demographic, diagnostic, and perioperative data were obtained for each patient. Additional retrospective chart review was performed to identify the patients with complete documentation that included both the 12 months prior to and post-TPIAT (n = 46). Patients without adequate documentation over the study periods were excluded from the analysis (n = 38). Clinical data were collected for each patient, which included hospital admissions, endoscopic procedures, narcotic and insulin requirements, and radiologic imaging over the entire study period.
Each MCCP patient included in this study underwent TPIAT as the initial surgical intervention for either (1) debilitating abdominal pain refractory to medical management or (2) recurrent, acute pancreatitis with documented enzyme elevations. MCCP was diagnosed based on imaging, functional testing, and patient symptomatology. Imaging included CT, endoscopic ultrasound (EUS), and endoscopic retrograde cholangiopancreatography (ERCP). Functional testing included measurement of fecal fat and elastase levels in conjunction with pancreatic bicarbonate levels. In most patients, diagnosis was made by EUS, ERCP, and CT findings. Confirmation with EUS required the presence of at least four or more of the nine conventional criteria: parenchymal findings included hyperechoic foci, hyperechoic stranding, hyperechoic lobularity, and cysts, while ductal findings included irregular duct contour, hyperechoic ductal margin, visible side branches, ductal calculi, and dilated main duct.12 If a patient had less than four Rosemont findings but a concomitant major finding (stones, honeycomb lobularity), then the patient was also diagnosed with MCP. Diagnosis confirmation by ERCP was based on the Cambridge classification.13 Patients with Cambridge II and III were designated as having MCCP. This study was approved by and conducted under the guidelines of the University of Cincinnati Institutional Review Board.
Cost-Effectiveness Modeling and Assumptions
A Markov health-state transition model was constructed to compare two strategies: (1) continued medical (nonsurgical) management of MCCP and (2) surgical intervention, comprised of TPIAT (Fig. 1a, b). The health states for each study cohort included the following four permutations of insulin and narcotic use: (1) insulin independent/narcotic independent (II/NI), (2) insulin independent/narcotic dependent (II/ND), (3) insulin dependent/narcotic independent (ID/NI), and (4) insulin dependent/narcotic dependent (ND/ID) and also death. In the surgical cohort, one additional health state was created to represent surgical intervention. The Markov model employed a 1-month cycle length, beginning at age 36 years (mean age of study cohort) and a lifetime horizon (defined as ending at age 100 years).
Patient intervention and outcome probabilities and rates were based on the University of Cincinnati MCCP patient data. Specifically in the surgical cohort, pertinent perioperative outcomes included perioperative mortality, complication rate, and readmission rate. In both cohorts, frequency and volume of three-dimensional imaging (CT), endoscopy (EGD, ERCP, and/or EUS), insulin use, and narcotic use were incorporated into the model. Means were utilized throughout the analysis to ensure all outcomes were incorporated, though not all data were precisely normally distributed. The model assumed that patients who did not undergo TPIAT would continue their rate of resource utilization (e.g., hospital admission, endoscopy use, etc.) indefinitely. In the surgical cohort, we also assumed that while health states and readmission rates might change within one postoperative year (as dictated by our data), neither health states nor readmission rates changed significantly 1 year after surgery.
Costs
Costs were estimated using 2013 Medicare payment in US$ (Table 1). Direct medical costs included surgeon and islet preparation fees, hospital admissions, and readmissions (DRG 439 for medical admissions and DRGs 405-407 for surgical admissions), narcotic use (in morphine equivalents, MEQs), insulin use, CT use, and endoscopic procedures (EGD, ERCP, and/or EUS). Hospital payment was based on no complications/comorbidities (without CC designation, DRG 407), minor complications (with CC designation; Clavien-Dindo 1-2, DRG 406), and major complications/comorbidities (with MCC designation; Clavien-Dindo 3-5, DRG 405). Costs of endoscopic procedures were calculated with a weighted average of the frequency of the individual components of all endoscopy procedures. Costs were discounted at 3 % per year, a standard practice to reflect the lessening value of the dollar over time.14
Survival and Quality of Life
Survival was calculated utilizing average annual mortality based on the US age-specific death rates as reported by the National Center for Health Statistics15 and excess mortality from the largest published series’ available for both medical and surgical management of chronic pancreatitis.16 – 18 From these rates, monthly transition probabilities of mortality were calculated for the Markov simulation. Final survival was reported in quality-adjusted life years (QALYs) after appropriate adjustment for quality of life (QOL). In the medical cohort, a QOL coefficient of 0.66 was used in the base case for patients who were either insulin or narcotic dependent, per published literature.19 Patients receiving narcotics and insulin were assumed to have a lower QOL than patients who were both narcotic and insulin independent (QOL coefficient 0.85 based on literature and expert opinion).20 , 21 In the surgical cohort, with similar differences between health states as noted in the medical cohort, the base case QOL coefficient reflected a 55 % improvement in QOL, based on literature examining long-term QOL outcomes in a surgically treated population.22 Based on the published literature, QOL was never assumed to be worse than ongoing medical management. Life expectancy also was discounted at 3 % per year.
Sensitivity Analysis
Sensitivity analyses were performed in a deterministic fashion. One-way sensitivity analysis was based on uncertainty or variability in the published literature that could potentially change the final cost and effectiveness profiles and conclusions. The parameters varied were QOL adjustment in the medical treatment strategy (QOL coefficient range 0.5–0.8), QOL adjustment in the surgical strategy (range 30–80 % improvement in QOL in comparison to QOL in medically treated patients), costs including surgical admission and complications (CC payment for no postoperative complications and MCC payment for all postoperative complications) and islet cell preparation ($10,000–$30,000; 50–200 % of our institutional cost), perioperative mortality (0–5 %), and long-term readmission rates in the medical treatment strategy (50 % below base case up to base case readmission rates).
Results
Patient Outcomes
Patient demographic information is listed in Table 2. The majority of patients were female (63.0 %, n = 29) and had either idiopathic (65.2 %, n = 30) or genetically linked (26.1 %, n = 12) chronic pancreatitis (CP). None of the patients had a previous pancreatic resection. Patients required daily narcotic pain medications at a mean of 138 MEQ/day. Only two patients required insulin prior to TPIAT.
All patients underwent a successful TPIAT, and there were no perioperative deaths. Operative details are listed in Table 3. The mean operative time of 549.5 min included tissue harvesting, total pancreatectomy, islet isolation and transplantation, and reconstruction. Mean hospital length of stay was 17 days. In this cohort, major complication rate after TPIAT was 30.4 % (n = 14). The initial postoperative health state distribution was as follows: insulin and narcotic independent (II/NI, n = 10), insulin independent and narcotic dependent (II/ND, n = 4), insulin dependent and narcotic independent (ID/NI, n = 21), and both insulin and narcotic dependent (ID/ND, n = 11).
Postoperative outcomes regarding pain management and glucose homeostasis are listed in Table 4. At 1 year after TPIAT, narcotic requirements decreased to 37.0 MEQ/day (p = 0.012), and 69.6 % of patients (n = 32) were narcotic independent. All patients achieved stable glycemic control after TPIAT, and 32.6 % of patients (n = 15) were insulin independent at 1 year.
Hospital admissions, days spent in the hospital, endoscopic procedures, and imaging events are depicted in Fig. 2. In the 12 months prior to TPIAT (medical management cohort), 46 patients accounted for a total of 78 hospital admissions and a total of 502 days in the hospital. Annual mean per patient endoscopic procedures was 1.4 (range = 0–6), and imaging events (CT/MRI) was 1.5 (range = 0–4). For the surgical cohort, the 30-day readmission rate was 37.0 %. There were no postoperative deaths in this cohort, and annual mean per patient admissions, endoscopic procedures, and imaging had decreased to 0.9 (range = 0–4), 0.4 (range = 0–2), and 0.9 (range 0–5), respectively.
Clinical events used to populate the Markov transition health-state model with data from both the medical management cohort (a) and surgical management cohort (b). Clinical variables captured include the number of hospital admissions and the number of days spent in the hospital, number of endoscopic procedures, and number of imaging events
Cost-Effectiveness and Sensitivity Analysis
Costs and quality-adjusted life expectancy for the medical management and surgical (TPIAT) management cohorts was $196,042/11.5 QALYs and $153,576/14.9 QALYs, respectively. Because the surgical strategy dominated the medical management strategy (lower costs and greater effectiveness), there was no incremental cost-effectiveness ratio (ICER) to calculate.
Results of sensitivity analyses are shown in Table 5. When QOL adjustment was made to reflect a 20 % increase/decrease in QOL above/below baseline, there was no change in cost (as expected), but quality-adjusted life expectancy ranged from 10.6 to 14.7 QALYs and 13.6 to 15.9 QALYs in the medical and surgical cohorts, respectively. Altering the relative improvement in QOL from 30 to 80 % in the surgically treated patients (in comparison to the continued medical therapy cohort) resulted in a range of 13.6–15.9 QALYs. Changing costs for patients without or with minor complications, payment for islet cell preparation, and perioperative mortality changed the cost profile by less than 10 % with no significant change in quality-adjusted survival. For each of these sensitivity analyses, the resultant cost and effectiveness profiles did not change the final conclusions—surgical therapy was always more effective at lower costs.
The one instance in which varying the model inputs did change the final cost-effectiveness comparison was found in changing the long-term readmission rates in the medical cohort. When rates of long-term readmission were ranged from the base case value to 50 % below baseline, the resultant cost in the medical cohort was $127,496. This scenario results in the surgical treatment strategy being more costly, though still with longer quality-adjusted survival and a resultant incremental cost-effectiveness ratio of $12,420/QALY.
Discussion
In the current study, we have examined the cost-effectiveness and resource utilization of TPIAT as compared to continued medical management in a population with MCCP. This analysis demonstrated that MCCP patients have a high burden of hospital admissions, days spent in the hospital, and diagnostic/therapeutic endoscopic procedures in addition to recurrent imaging events. Perhaps, the most profound finding was that in a medially managed cohort, a total of 502 days were spent in the hospital, with 78 hospital admissions over a 12-month time period. In the surgical cohort, there were a high number of readmissions in the immediate months following TPIAT; however, this burden was significantly reduced 6 months after TPIAT. Populating a Markov transition health-state model with data from both the medical management and surgical management cohorts, we were able to demonstrate that TPIAT is associated with both improved quality-adjusted survival but also reduced lifelong medical costs.
This study represents the first cost-effectiveness analysis comparing traditional medical management of MCCP to primary surgical intervention with TPIAT. Traditionally, medical management has been based on the notion that MCCP can only be treated medically, with no meaningful surgical intervention available for this patient population. Because these patients are not amenable to drainage procedures typically utilized for large-duct disease, therapeutic options have been quite limited. However, more recent publications have demonstrated persistent pain despite medical therapy over 10-year follow-up, and clearly, more effective treatment options are needed.4 , 23 In fact, a study by Nealon and Thompson demonstrated that in a patient cohort of 56 patients with nondilated duct CP, only one patient experienced spontaneous pain relief.4 Since that time, numerous case series have reported improved symptoms after surgical intervention, and only one treatment—total pancreatectomy—completely removes the inciting organ and source of the symptoms. With advances in islet cell autotransplantation, TPIAT has become a viable option with improved pain relief, stable glycemic control, and improved QOL.16 , 22 , 24 , 25 Only, one previous study from Leicester26 has examined the cost of TPIAT. They showed equivocal results when comparing TPIAT and medical management however did not account for quality-adjusted survival in this analysis.26 Findings of our current study add to the evidence favoring TPIAT with improved health care costs and prolonged quality-adjusted survival.
The limitations of this study must be recognized. First, the patient utilities used for QOL adjustment are not uniformly reported and consistently used across study populations. As such, QOL adjustments for both medical and surgical cohorts were independently populated, based on the largest, highest quality literature available. It is unfortunate that such QOL measurements are not more standardly gathered and reported, as consistency within a disease state would make all subsequent comparative studies more interpretable and of higher integrity. However, based on both the significant cost savings and the sensitivity analyses incorporating a wide range of QOL adjustments in the surgical cohort (including the extremely unlikely notion of no improvement in QOL after TPIAT), the conclusions in this study would not change; TPIAT is more cost-effective than medical management for MCCP. Secondly, we do not have lifelong data for these patients, and the assumptions about resource utilization and QOL may not hold true years after the index operation. Our group is currently accumulating patient-specific outcomes for patients 5 years after surgery, and beyond, which will provide increasingly accurate data for future modeling.
Lastly, our cost estimates are based on Medicare reimbursement, and this facet of our study deserves special attention. While publicly available and verifiable, these payments do not reflect reimbursements of all age groups and insurance types represented in our patient sample, and the absolute dollar value of costs reported here is likely to be low. However, the costs ascribed to patients in this study are consistent between study cohorts, and again, the sensitivity analysis demonstrates that our conclusions would not change, even with wide variability in payment for individual components of our model. Furthermore, Medicare payments are almost certainly significantly underestimated relative to payments made by private insurers. If payment amounts of private insurers were available for this study, it is quite likely that the absolute cost savings of TPIAT would be more pronounced. For example, if hospital costs for an acute exacerbation of chronic pancreatitis were reimbursed at a rate of $15,000 per admission instead of approximately $5,000 (Medicare payment), the compounding excess costs of readmission associated with medical management would make TPIAT even more desirable from a cost-effectiveness perspective.
Conclusion
In summary, this study further validates the early use of TPIAT as a first-line surgical therapy in patients with refractory, minimal change CP. There is a high burden of medical care devoted to patients with MCP managed with traditional medical therapies. TPIAT is associated with an initial upfront cost and early postoperative admission burden, but when compared to medical management over a patients’ expected lifetime, TPIAT is associated with decreased cost and increased quality-adjusted survival. These findings suggest that tertiary care centers should look to expand the use of TPIAT in this patient population and that the initial costs and risks of TPIAT are well justified.
References
Warshaw AL, Banks PA, Fernandez-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998;115:765-776.
Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992;33:1566-1571.
Shrikhande SV, Kleeff J, Friess H, Buchler MW. Management of pain in small duct chronic pancreatitis. J Gastrointest Surg 2006;10:227-233.
Nealon WH, Thompson JC. Progressive loss of pancreatic function in chronic pancreatitis is delayed by main pancreatic duct decompression. A longitudinal prospective analysis of the modified puestow procedure. Ann Surg 1993;217:458-466; discussion 466-458.
Izbicki JR, Bloechle C, Broering DC, Kuechler T, Broelsch CE. Longitudinal V-shaped excision of the ventral pancreas for small duct disease in severe chronic pancreatitis: prospective evaluation of a new surgical procedure. Ann Surg 1998;227:213-219.
Izbicki JR, Bloechle C, Knoefel WT, Rogiers X, Kuechler T. Surgical treatment of chronic pancreatitis and quality of life after operation. Surg Clin North Am 1999;79:913-944.
Traverso LW, Kozarek RA. Pancreatoduodenectomy for chronic pancreatitis: anatomic selection criteria and subsequent long-term outcome analysis. Ann Surg 1997;226:429-435; discussion 435-428.
Hutchins RR, Hart RS, Pacifico M, Bradley NJ, Williamson RC. Long-term results of distal pancreatectomy for chronic pancreatitis in 90 patients. Ann Surg 2002;236:612-618.
Rios GA, Adams DB, Yeoh KG, Tarnasky PR, Cunningham JT, Hawes RH. Outcome of lateral pancreaticojejunostomy in the management of chronic pancreatitis with nondilated pancreatic ducts. J Gastrointest Surg 1998;2:223-229.
Yekebas EF, Bogoevski D, Honarpisheh H, Cataldegirmen G, Habermann CR, Seewald S, Link BC, et al. Long-term follow-up in small duct chronic pancreatitis: A plea for extended drainage by “V-shaped excision of the anterior aspect of the pancreas. Ann Surg 2006;244:940-946; discussion 946-948.
Sutton JMS, Nathan; Sussman, Jeffrey J; Smith, Milton; Kurland, Jayde E; Brunner, John E; Salehi, Marziah; Choe, Kyuran A; Ahmad, Syed A. Total pancreatectomy and islet cell autotransplantation as a means of treating patients with genetically linked pancreatitis. Surgery 2010;48:676-685.
Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009;69:1251-1261.
Vitale GC, Davis BR, Zavaleta C, Vitale M, Fullerton JK. Endoscopic retrograde cholangiopancreatography and histopathology correlation for chronic pancreatitis. Am Surg 2009;75:649-653; discussion 653.
West RR, McNabb R, Thompson AG, Sheldon TA, Grimley Evans J. Estimating implied rates of discount in healthcare decision-making. Health Technol Assess 2003;7:1-60.
2010 Census Data.
Sutherland DER, David M; Bellin, Melena D, Hering, Bernard J; Beilman, Gregory J; Dunn, Ty B; Chinnakotla, Srinath; Vickers, Selwyn M; Bland, Barbara, Balamurugan, AN; Freeman, Martin L; Pruett, Timothy L. Total Pancreatectomy and Islet Autotransplantation for Chronic Pancreatitis. J AM Coll Surg 2012:409-426.
Nojgaard C, Bendtsen F, Becker U, Andersen JR, Holst C, Matzen P. Danish patients with chronic pancreatitis have a four-fold higher mortality rate than the Danish population. Clin Gastroenterol Hepatol 2010;8:384-390.
Jupp J, Fine D, Johnson CD. The epidemiology and socioeconomic impact of chronic pancreatitis. Best Pract Res Clin Gastroenterol 2010;24:219-231.
Wassef W, DeWitt JM, Wilcox M, Whitcomb DC, Yadav D, Amann S, Mishra G, et al. Pancreatitis Quality of Life Instrument (PANQOLI): A Psychometric Evaluation. American College of Gastroenterology 2012 Annual Scientific Meeting Abstracts 2012;60.
Pezzilli R, Morselli-Labate AM, Fantini L, Campana D, Corinaldesi R. Assessment of the quality of life in chronic pancreatitis using Sf-12 and EORTC Qlq-C30 questionnaires. Dig Liver Dis 2007;39:1077-1086.
Wehler M, Nichterlein R, Fischer B, Farnbacher M, Reulbach U, Hahn EG, Schneider T. Factors associated with health-related quality of life in chronic pancreatitis. Am J Gastroenterol 2004;99:138-146.
Walsh RM, Saavedra JR, Lentz G, Guerron AD, Scheman J, Stevens T, Trucco M, et al. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg 2012;16:1469-1477.
Lankisch PG, Lohr-Happe A, Otto J, Creutzfeldt W. Natural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion 1993;54:148-155.
Morgan K, Owczarski SM, Borckardt J, Madan A, Nishimura M, Adams DB. Pain control and quality of life after pancreatectomy with islet autotransplantation for chronic pancreatitis. J Gastrointest Surg;16:129-133; discussion 133-124.
Clayton HA, Davies JE, Pollard CA, White SA, Musto PP, Dennison AR. Pancreatectomy with islet autotransplantation for the treatment of severe chronic pancreatitis: the first 40 patients at the Leicester General Hospital. Transplantation 2003;76:92-98.
Garcea G, Pollard CA, Illouz S, Webb M, Metcalfe MS, Dennison AR. Patient satisfaction and cost-effectiveness following total pancreatectomy with islet cell transplantation for chronic pancreatitis. Pancreas 2013;42:322-328.
Conflict of Interest
No grant support or other assistance to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Jeffrey B Matthews (Chicago, IL): Total pancreatectomy with islet autotransplantation has emerged as an important option for selected patients incapacitated by recurrent acute or chronic pancreatitis. TPIAT can be highly effective for the right patient. Yet, many insurers still view islet autotransplantation as experimental and do not offer coverage. Your work adds important information regarding cost-effectiveness of TPIAT versus continued best medical therapy.
I have two questions:
You use the term “minimal change disease,” but most of your patients met EUS criteria or had other imaging evidence for small duct chronic pancreatitis. If minimal change disease exists at all, it refers to the patient with “pancreatic” type pain but an otherwise normal pancreatic duct and subthreshold EUS and imaging criteria for parenchymal disease. Using that definition, did you have any true minimal change patients, and if so, how did you confirm that the pancreas was the source of the pain, as opposed to visceral hyperalgesia or functional abdominal pain syndromes meeting Rome III criteria?
Second, you analyzed the subset of small duct patients who were in the worst shape, the ones with high narcotic use, frequent hospitalizations, nutritional support, and imaging. Do you believe that we should be offering TPIAT more liberally or earlier or for patients with large duct disease that might otherwise be approached endoscopically or by conventional surgery? Because hospital admission is the key driver of costs, what strategies can keep patients out of ERs and avoid admission, tipping the balance back in favor of continued medical therapy?
Closing Discussant
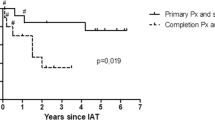
Dr. Wilson: Thank you Dr. Matthews for the comments and questions. At our institution, all chronic pancreatitis patients undergo a multidisciplinary evaluation involving gastroenterology, radiology, and surgery. This multidisciplinary approach is one of the foundations for managing these patients because as you point out, distinguishing chronic pancreatitis from visceral hyperalgesia and functional abdominal pain syndromes is critical for successful treatment. The diagnosis of chronic pancreatitis is based on patient symptomatology and confirmed with imaging and/or functional testing. The patients included in this evaluation are not the typical chronic pancreatitis patients with calcific large duct disease. Although all patients in our series did undergo CT scans and a subset also underwent ERCP, the majority were diagnosed with a combination of EUS and pancreas function testing with secretin. The term “small duct disease” and “minimal change pancreatitis” are often used interchangeably and the exact definition of each has not clearly been defined. However, at the University of Cincinnati, we prefer to use “minimal change pancreatitis” to define this subgroup of patients. Our criteria to consider surgery is having at least four out of nine EUS Rosemont features or having less than three, but one has to be a major finding such as honeycomb lobularity. These patients are not amenable to traditional resection or decompressive procedures and therefore at our institution are offered TPIAT as an initial surgical intervention. We do not believe this strategy should be extrapolated to those patients with calcific or large-duct disease as the initial treatment modality. Based on our clinical experience, the majority of patients with calcific or large-duct disease are successfully managed with traditional resection or decompressive procedures. In our experience, approximately 10 % of patients that undergo resection or decompression as the initial surgical treatment have disease progression. These patients are then reevaluated as possible candidates for completion pancreatectomy with islet cell autotransplantation.
Our cost effectiveness model identifies hospital admissions as the key driving factor in the cost of medical management. In our sensitivity analysis, reducing hospital admissions in the medical arm by 50 % results in lower lifetime medical costs to the patient, though is still associated with worse long-term quality-adjusted survival. Strategies to keep patients out of the hospital have been attempted and have focused on providing similar care (parenteral/enteral nutrition, intravenous fluids, narcotic pain medications, etc.) in the outpatient clinic or even the patient’s home. While these strategies may prevent recurrent hospital admissions in some patients, they do not address the poor quality of life that these patients experience.
Rights and permissions
About this article
Cite this article
Wilson, G.C., Ahmad, S.A., Schauer, D.P. et al. Cost-Effectiveness of Total Pancreatectomy and Islet Cell Autotransplantation for the Treatment of Minimal Change Chronic Pancreatitis. J Gastrointest Surg 19, 46–55 (2015). https://doi.org/10.1007/s11605-014-2612-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2612-3