Abstract
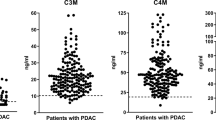
Type VI collagen (COL6) forms a microfibrillar network often associated with type I collagen and constitutes a major component of the desmoplastic reaction in pancreatic ductal adenocarcinoma (PDA). We have demonstrated recently that the α3 chain of COL6, COL6A3, is highly expressed in PDA tissue and undergoes tumor-specific alternative splicing. In this study, we investigated the diagnostic value and clinical significance of circulating COL6A3 protein and mRNA in PDA. COL6A3 levels in sera from patients with PDA (n = 44), benign lesions (n = 46) and age-matched healthy volunteers (n = 30) were analyzed by enzyme-linked immunosorbent assays (ELISA). Predictive abilities of COL6A3 were examined using receiver operating characteristic (ROC) curves from logistic regression models for PDA versus normal or benign serum levels. Expression levels were correlated with clinicopathological parameters. Real-time PCR was used to analyze the presence of COL6A3 mRNA containing alternative spliced exons E3, E4, and E6. Circulating COL6A3 protein levels were significantly elevated in PDA patients when compared to healthy sera (p = 0.0001) and benign lesions (p = 0.0035). The overall area under the ROC was 0.975. Log(COL6A3) alone provided good discrimination between PDA and benign lesions (area under the curve (AUC) = 0.817), but combined with CA19-9 provided excellent discrimination (AUC = 0.904). Interestingly, high COL6A3 serum levels were significantly associated with perineural invasion and cigarette smoking. Combined E3, E4, and E6 serum RNA values provided good sensitivity but low specificity. Our data demonstrate for the first time the potential clinical significance of circulating COL6A3 in the diagnosis of pancreatic malignancy.




Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012: 62:10–29.
Hidalgo M. Pancreatic cancer. N Engl J Med 2010: 362:1605–1617.
Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol 2008: 3:157–188.
Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol 2013: 107:15–22.
Mahadevan D, Von Hoff DD. Tumor–stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2007: 6:1186–1197.
Chu ML, Zhang RZ, Pan TC et al. Mosaic structure of globular domains in the human type VI collagen alpha 3 chain: similarity to von Willebrand factor, fibronectin, actin, salivary proteins and aprotinin type protease inhibitors. EMBO J 1990:9(2): 385–393.
Arafat H, Lazar M, Salem K et al. Tumor-specific expression and alternative splicing of the COL6A3 gene in pancreatic cancer. Surgery 2011: 150:306–315.
Chang KC, Lu YC, Lin MJ, Chen HY, Jin YT. Desmoplastic tumour-associated stroma versus neural tissue in central nervous system metastasis: effects of different microenvironments on tumour growth. Histopathology 2011: 59:31–39.
Lohi J, Leivo I, Oivula J, Lehto V, Virtanen I. Extracellular matrix in renal cell carcinomas. Histol Histopathol 1998: 13:785–796.
Sethi T, Rintoul RC, Moore SM et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med 1999: 5:662–668.
Sherman-Baust CA, Weeraratna AT, Rangel L et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer cell 2003: 3:377–386.
Ohlund D, Lundin C, Ardnor B, Oman M, Naredi P, Sund M. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br J Cancer 2009: 101:91–97.
Mazouni C, Arun B, Andre F et al. Collagen IV levels are elevated in the serum of patients with primary breast cancer compared to healthy volunteers. Br J Cancer 2008: 99:68–71.
Ohlund D, Franklin O, Lundberg E, Lundin C, Sund M. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer (2013). DOI: 13:154-2407-13-154.
Shields MA, Dangi-Garimella S, Krantz SB, Bentrem DJ, Munshi HG. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. J Biol Chem 2011: 286:10495–10504. (duplicate of ref. 11)
Sherman-Baust CA, Weeraratna AT, Rangel L et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer cell 2003: 3:377–386.
Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas 2004: 28:38–44.
Korc M. Pancreatic cancer-associated stroma production. Am J Surg 2007: 194:S84-S86.
Lynch SM, Vrieling A, Lubin JH et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol 2009: 170:403–413.
Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Practice and Research: Clinical Gastroenterology 2006: 20:197–209.
Heeschen C, Jang JJ, Weis M et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 2001: 7:833–839.
Bose C, Zhang H, Udupa KB, Chowdhury P. Activation of p-ERK1/2 by nicotine in pancreatic tumor cell line AR42J: effects on proliferation and secretion. Am J Physiol Gastrointest Liver Physiol 2005: 289:G926-34.
Chowdhury P, Bose C, Udupa KB. Nicotine-induced proliferation of isolated rat pancreatic acinar cells: effect on cell signalling and function. Cell Prolif 2007: 40:125–141.
Lazar M, Sullivan J, Chipitsyna G et al. Involvement of osteopontin in the matrix-degrading and proangiogenic changes mediated by nicotine in pancreatic cancer cells. J Gastrointest Surg 2010: 14:1566–1577.
Mahadevan D, Von Hoff DD. Tumor–stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2007: 6:1186–1197.
Makareeva E, Han S, Vera JC et al. Carcinomas contain a matrix metalloproteinase-resistant isoform of type I collagen exerting selective support to invasion. Cancer Res 2010: 70:4366–4374.
Stallings-Mann M, Radisky D. Matrix metalloproteinase-induced malignancy in mammary epithelial cells. Cells Tissues Organs 2007: 185:104–110.
Zhou J, Olson BL, Windsor LJ. Nicotine increases the collagen-degrading ability of human gingival fibroblasts. J Periodontal Res 2007: 42:228–235.
Heeschen C, Jang JJ, Weis M et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 2001: 7:833–839.
Tipton DA, Dabbous MK. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblasts in vitro. J Periodontol 1995: 66:1056–1064.
Singh S, Tang SJ, Sreenarasimhaiah J, Lara LF, Siddiqui A. The clinical utility and limitations of serum carbohydrate antigen (CA19-9) as a diagnostic tool for pancreatic cancer and cholangiocarcinoma. Dig Dis Sci 2011: 56:2491–2496.
Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol 2013: 107:15–22.
Yang S, Shin J, Park KH et al. Molecular basis of the differences between normal and tumor tissues of gastric cancer. Biochimica et biophysica acta. Molecular basis of disease 2007: 1772:1033–1040
Thorsen K, Sorensen KD, Brems-Eskildsen AS et al. Alternative splicing in colon, bladder, and prostate cancer identified by exon array analysis. Mol Cell Proteomics 2008: 7:1214–1224.
Farkas DH, Kaul KL, Wiedbrauk DL, Kiechle FL. Specimen collection and storage for diagnostic molecular pathology investigation. Arch Pathol Lab Med 1996: 120:591–596.
Kopreski MS, Benko FA, Kwak LW, Gocke CD. Detection of tumor messenger RNA in the serum of patients with malignant melanoma. Clin Cancer Res 1999: 5:1961–1965.
Hunt JL. Molecular pathology in anatomic pathology practice: a review of basic principles. Arch Pathol Lab Med 2008: 132:248–260.
Acknowledgments
This work was supported by a grant from the University City Science Center QED award and the PA Commonwealth Keystone Innovation Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Syed Ahmad (Cincinnati, Ohio): Christopher, I want to congratulate you, Dr. Arafat, Dr. Yeo and your co-authors on a very nice presentation and novel work. I also want to thank you for sending me the manuscript in advance of the meeting. Your work suggests that the alpha 3 chain of the type VI collagen may be utilized as a novel biomarker for diagnosing pancreatic cancer. With this background, I would like to remind everyone on the potential characteristics of a useful biomarker, they should: (1) have specific production by premalignant or malignant tissue early in the progression of disease; (2) be produced at detectable levels in all patients with a specific malignancy; (3) they should be expressed in an organ site-specific manner; (4) their levels should be related quantitatively to tumor volume, biological behavior, or disease progression; (5) they should have a relatively short half-life, reflecting temporal changes in tumor burden and response to therapy; (7) there should be a standardized and reproducible assay for measuring the biomarker; and finally, for screening, (8) they should have relatively high sensitivity and very high disease specificity, i.e., to yield a very low false-positive rate (FPR) and a reasonable low false-negative rate (FNR).
Based on these characteristics, I have a few questions:
1. You evaluated 46 patients with IPMN and benign cystic lesions, do you have any data on the production of COL6A3 in premalignant tissue. Does the level rise from premalignant to malignant disease or is COL6A3 only elevated in pancreas cancer? Does COL6A3 correlate with prognosis or recurrence?
2. Are levels elevated in other types of cancer or benign inflammatory states?
3. How do you explain the lack of increase in levels of COL6A3 from less aggressive to more aggressive clinical-pathologic factors? Studies would suggest that more aggressive cancers have more of a desmoplastic response and I would have expected increasing levels?
4. Please give us insight into the false-positive and false-negative rate for this biomarker?
5. Your data would suggest that, by itself, COL6A3 is equivalent to CA 19-9 in predicting the presence of cancer around 81 %. This increases to 90 % when the two are combined. How is this biomarker different than others that have been reported to increase the PPV of CA 19-9?
Once again, I thank you for the opportunity to review this paper and I look forward to hearing your answers.
Closing Discussant
Dr. Christopher Kang: Thank you Dr. Ahmad for the insightful and thought-provoking comments and questions. In regards to the levels of COL6A3 from normal to premalignant and malignant disease, the levels of serum COL6A3 were elevated with statistical significance in premalignant lesions compared to normal as seen with our IPMN and cystadenomas. From premalignant to malignant lesions, there was also a statistical significant increase in COL6A3 levels. Although there was no association with statistical significance between survival and levels of COL6A3, there was a trend with patients with low levels of COL6A3 having better survival.
There have not been any studies from our lab or in the literature review that show serum COL6A3 levels in other cancers or inflammatory states. We would like to examine the levels in other cancers and inflammatory states in a bigger study. Chronic pancreatitis would be especially interesting.
Actually, our data show significant association of high COL6A3 levels with perineural invasion and with cigarette smoking. This implies a role for COL6A3 in the metastatic behavior of PDA cells. We are currently exploring the functions of the individual isoforms that we showed to be high in the malignant lesions. However, the lack of increase in COL6A3 levels in more advanced stages or larger masses can be explained by the fact that COL6A3 itself has no effect on cell proliferation (unpublished observation). With actual functional studies using PDA cell lines, studies that are currently ongoing will be able to elucidate the functional aspects of this intriguing stromal protein.
As we have seen with CA 19-9, differing the cutoff levels of expression can change false-positive and false-negative rate. We have not determined a true cutoff value for COL6A3 as of yet, but with more samples and including the other pathologies, we may achieve a true cutoff value.
In response to your final question, from my review of the literature regarding CA19-9 and other biomarkers improving PPV, a common theme is great initial results; however, there is a failure of reproducibility. We have reproduced our results and believe when we synthesize antibodies to COL6A3 isoforms, we will have a true diagnostic biomarker that follows the characteristics you have mentioned.
Rights and permissions
About this article
Cite this article
Kang, C.Y., Wang, J., Axell-House, D. et al. Clinical Significance of Serum COL6A3 in Pancreatic Ductal Adenocarcinoma. J Gastrointest Surg 18, 7–15 (2014). https://doi.org/10.1007/s11605-013-2326-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-013-2326-y