Abstract
Background
Recurrent Clostridium difficile colitis is common, yet the ability to predict recurrence is poorly developed.
Methods
Patients ≥18 years of age treated at our institution for C. difficile of any severity were consecutively enrolled. C. difficile colitis was defined as symptoms of colitis with a positive PCR stool test. Each bacterial isolate was studied for virulence factors: tcdC mutations via PCR; the presence of genes for toxins A, B, and binary toxin using restriction fragment length polymorphism; and identification of ribotype 027 by PCR. Chi-squared tests, t tests, and logistic and linear regression were used to determine which virulence factors predicted recurrence.
Results
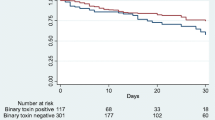
Sixty-nine patients (male, 57 %) were studied, with a mean age of 64 ± 13 years. Twenty-one (30 %) patients were initially diagnosed as outpatients. There was no difference (p > 0.05) between virulence factors among inpatients and outpatients. The presence of a binary toxin gene was the single virulence factor independently associated with recurrence (p = 0.02). The combination of a tcdC mutation with binary toxin gene resulted in the highest odds of recurrence (OR, 5.3; 95 % CI, 3.52–6.09).
Conclusion
Binary toxin gene is a predictor of recurrent infection. Its presence may require longer antibiotic regimens in an effort to lower already elevated recurrence rates.
Similar content being viewed by others
References
Bartlett JG, Perl TM. The new Clostridium difficile—what does it mean? N Engl J Med 2008;353(23):2503-05.
Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg 2002;235(3):363-372.
Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 2004;171(5):466-472.
Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005;366:1079-84.
McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005;353:2433-41.
McFarland LV, Surawicz CM, Rubin M, et al. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999;20:43-50.
McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002;97(7):1699-75.
McFarland LV, Surawicz CM, Rubin M, Fekety R, et al. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999;43-50.
Jabbar U, Leischner J, Kasper D, Gerber R, et al. Effectiveness of alcohol-based hand rubs for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemol 2010;31(6):565-70.
Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011;53(1):42-8.
FDA U.S. Food and Drug Administration. Accessed on 4/2/2012; http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm290838.htm
Rupnik M, Avesani V, Janc M, von Eichel-Streiber C, Delmée M. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J Clin Microbiol 1998;36(8):2240-7.
Stubbs SL, Brazier JS, O’Neill GL, Duerden BI. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J Clin Microbiol 1999;37:461-63.
Spigaglia P, Mastrantonio P. Molecular analysis of the pathogenicity locus and polymorphism in the putative negative regulator of toxin production (TcdC) among Clostridium difficile clinical isolates. J Clin Microbiol 2002;40(9):3470-5.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83.
Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431-55.
Popoff MR, Rubin E, Gill DM, Boquet P. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect Immun 1988;56:2299-306.
Thelestram M, Chaves-Olarte E. Cytotoxic effects of the Clostridium difficile toxins. Cur Top Microbiol Immunol 2000;250:85-96.
Rupnik M, Grabnar M, Geric B. Binary toxin producing Clostridium difficile strains. Anaerobe 2003;9:289-294.
Barth H, Aktories K, Popoff MR, Stiles BG, et al. Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus mechanism of ADP ribosylating toxins. FEBS J 2006;273:4579-4593.
Sundriyal A, Roberts AK, Ling R, McGlashan J, et al. Expression, purification and cell cytotoxicity of actin-modifying binary toxin from Clostridium difficile. Protein Expr Purif 2010;74:42-48.
Vandekerckhove J, Schering B, Barmann M, et al. Clostridium perfringens iota toxin ADP ribosylates skeletal muscle actin in Arg-177. FEBS Lett 1987(225):48-52.
Reuner KH, Presek P, Boschek CB, et al. Botulinum C2 toxin ADP-ribosylates actin and disorganizes the microfilament network in intact cells. Eur J Cell Biol 1987;43:134-140.
Davies AH, Roberts AK, Shone CC, Acharya KR. Super toxins from a super bug: structure and function of Clostridium difficile toxins. Biochem KJ 2011;436:517-526.
Geric B, Johnson S, Gerding DN, Grabnar M et al. Frequency of binary toxin genes among Clostridium difficile strains that do not produce large clostridial toxins. J Clin Microbiol 2003;41:5227-32.
Stubbs S, Rupnik M, Gilbert M, Brazier J, et al. Production of actin specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol Lett 2000;186:307-312.
Goldenberg AD, French GL. Lack of association of tcdC type and binary toxin status with disease severity and outcome in toxigenic Clostridium difficile. J Infect 2011;62(5):355-362.
McCarthy DM. Adverse effects of proton pump inhibitor drugs: clues and conclusions. Curr Opin Gastroenterol 2010;26:624-631.
Naggie S, Miller BA, Zuzak KB, et al. A case-control study of community-associated Clostridium difficile infection: no role for proton pump inhibitors. Am J Med 2011;124(3):276.e1-276.e7.
Kim YG, Graham DY, Jang BI. Proton pump inhibitor use and recurrent Clostridium difficile-associated disease. J Clin Gastroenterol 2012;46(5):397-400.
Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005;294:2989-95.
Dupuy B, Govind R, Antunes A, Matamouros S. Clostridium difficile toxin synthesis is negatively regulated by TcdC. J Med Microbiol 2008;57:685-689.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Sekar Dharmarajan (St. Louis, MO): Thank you Mr. Chairman. While the surgical literature is replete with studies that correlate clinical patient factors with morbidity, need for surgery, and mortality from C. difficile infection, Dr. Stewart and colleagues have taken the novel and thought-provoking approach of characterizing bacterial virulence factors and correlating these with morbidity from C. difficile in the form of recurrent infection. I have three broad areas of comments/questions.
The first surrounds the definition of recurrent C. difficile colitis. The authors define recurrent C. difficile infection as two consecutive positive C. difficile stool samples no closer than 21 days apart. Without a negative intervening sample, how do we know this is recurrent C. difficile infection as opposed to persistent C. difficile infection, as we know that C. difficile is notoriously difficult to eradicate? Specifically, do you have any data on how far apart temporally the positive stool samples were in the patients with recurrent C. difficile? More interestingly, do you have any data on the bacteriology of the recurrent C. difficile isolates to see how they are compared to the original isolate?
The second area is with regard to the broader generalizability or applicability of the study. As the authors state in their manuscript, the prevalence of the binary toxin gene in this study was five- to tenfold higher than that previously reported in the literature. Similarly, ribotype 027, which has been found in previous studies to be hypervirulent, was not associated with any morbidity or mortality in the present study. Is it possible that, while binary toxin is the key to predicting recurrent infection at Hershey, these factors may differ institution to institution?
Finally, the last area is with regard to patient or host factors that certainly must contribute to morbidity and mortality from C. difficile infection. While the present study found no clinical factors that predicted recurrent infection, I wonder if the authors have plans or could comment on studying the interaction of patient genotypes at genetic loci that may render susceptibility to infection with bacterial virulence factors on morbidity from C. difficile. Similarly, as the authors comment in their manuscript, the host microbiome has become an increasingly important area of research in determining outcomes from a variety of disease processes, and I wonder if the authors had any comment on their plans to study this.
Thank you for the opportunity to review your extremely well-written manuscript.
Selection Bias: There is no patient with fulminant C. difficile colitis as defined by need for surgery or death.
Closing Discussant
Dr. David B. Stewart: I would like to thank the SSAT for the opportunity to present our research, and I would also like to express my gratitude to Dr. Dharmarajan for being willing to serve as a discussant for our presentation.
Dr. Dharmarajan’s first point is perhaps the most critical issue which he raised as a discussant. There are no consensus-based definitions, for all research endeavors on the topic, which define recurrent C. difficile colitis (RCDC) in any scientifically meaningful manner. This lack of standardization would potentially allow for an inflated estimate of RCDC, if the time interval between two consecutive CDC episodes was diminished to a great enough degree. In fact, in reviewing the literature which deals with recurrent C. difficile, the reader will encounter both heterogeneity in the definition of recurrence as well as a degree of arbitrariness which includes extremely short time intervals (<10 days) as well as criteria that range from clinical symptoms of diarrhea to tests confirming the presence of toxigenic C. difficile. In our case, it seemed appropriate to set the definition of RCDC at a point at least far enough after the diagnosis of the index episode to allow for the standard course of 10–14 days of antibiotics to be completed. We then allowed for an additional week of time to elapse in order to better ensure that we avoided collapsing recurrence into persistence. While our approach is not universal in acceptance, we believe it is fair and that it has a conceptual appeal given what we have described. Further, our recurrence rates fall into the same general range as those previously published in larger studies.
There are no data available to currently guide the definition of when persistence ends and recurrence begins, and so there are no data available to answer the issue of temporal relationships for persistence or recurrence. In part, this very relevant question will not be answered until we have large C. difficile registries that can provide, at minimum, regional epidemiologic information regarding endemic ribotypes and virulence characteristics. Such resources do not exist in this country, though they do exist in seminal forms in Canada.
The issue related to whether a recurrent episode of CDC is due to a different ribotype is something our group will be focusing on in the future. There are no relevant data on this topic currently available, though the issue is critical. Our group has unpublished data which demonstrate that C. difficile responds to drugs such as proton-pump inhibitors in a ribotype-dependent manner, such that some ribotypes will produce more toxins in the presence of PPIs and some will express toxin genes to a lesser degree. Some are actually unaffected by PPIs. If this is confirmed in larger studies, it will introduce an additional complexity in understanding C. difficile and in how environmental cues, including the drugs we use to eradicate the organism, may lead to different clinical outcomes. There is a biologic basis for such phenomena, and in the same manner that we push for personalized medicine with respect to diseases like cancer, we need personalized microbiology to a much greater degree than is presently available.
The second comment deals with whether those bacterial genetic signatures that were associated with higher rates of recurrence in our study might not be relevant in a different population of C. difficile. Until our findings are prospectively evaluated in a multiregional fashion, then this concern will not be definitively addressed. However, the idea that binary toxin would negatively affect clinical outcomes in CDC has scientific plausibility, given that previous reports have suggested that this is a more potent form of toxin.
Dr. Dharmarajan’s last comment deals with how host factors, including other microorganisms within the gut, may influence the behavior of C. difficile. The issue of the gut microbiome is certainly the hot topic of our day in microbiology, and for good reason. However, our understanding of the microbiome concept is currently so piecemeal that the more we learn, the less we know. There is at least one publication from several years ago which demonstrated a higher rate of RCDC when the human subject was found to harbor certain single nucleotide polymorphisms in the gene for IL-8, though this publication used a definition for recurrence, which may have lent toward artificially higher recurrence rates. Our own group is now using metabolomics to study how different environmental elements within the gut, such as varying levels of electrolytes and micronutrients, might affect transcriptional control of virulence genes in C. difficile. We have also begun obtaining rectal swabs on CDC patients in order to study the mRNA present in the organism at the time the sample is obtained, in an effort to study the “transcriptome” and how differential gene expression is associated with clinical outcomes such as recurrence, fulminant colitis, and death from CDC.
One final comment—these were consecutively enrolled patients which were presented in our study. Though none of our study patients required colectomy or died, there were patients with severe CDC based on the IDSA classification schema. Surgical intervention for CDC, while much more common than in previous eras, is still relatively infrequent, which makes studying this particular patient group more difficult when attempting to obtain informed consent for a stool sample is required and when the patient is critically ill.
Rights and permissions
About this article
Cite this article
Stewart, D.B., Berg, A. & Hegarty, J. Predicting Recurrence of C. difficile Colitis Using Bacterial Virulence Factors: Binary Toxin Is the Key. J Gastrointest Surg 17, 118–125 (2013). https://doi.org/10.1007/s11605-012-2056-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-012-2056-6