Abstract
Background
Positive peritoneal cytology confers the same prognosis as clinical stage IV disease in gastric cancer. Conventional cytology examination, however, has low sensitivity. We hypothesize that real-time polymerase chain reaction (RT-PCR) may have increased sensitivity and provide more accurate staging information.
Methods
From February 2007 to April 2009, peritoneal lavage samples were collected prospectively from 156 patients with biopsy-proven gastric cancer undergoing staging laparoscopy. These washings were analyzed by both Papanicolaou staining and RT-PCR for the tumor marker carcinoembryonic antigen (CEA).
Results
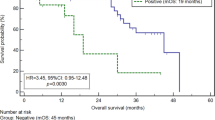
Visible peritoneal disease was seen at laparoscopy in 38 patients (LAP+, 24%). Cytology was positive (CYT+) in 23 patients, while RT-PCR was positive (PCR+) in 30. The sensitivity of CYT for the detection of visible disease was 61% compared to 79% for PCR (P = 0.02). No visible peritoneal disease was seen at laparoscopy (LAP−) in 118 (76%) patients. Eight (7%) were CYT+, while 28 (24%) were PCR+. Predictors of PCR positivity included advanced-stage disease (T3–4 vs. T1–2 tumors) and poor pathologic features such as vascular or perineural invasion. Long-term follow-up demonstrated a worse survival of LAP−CYT−PCR+ (P = 0.0003) and LAP−CYT+PCR+ (P = 0.0004) compared to LAP−CYT−PCR− patients. There was no significant difference in survival between CYT−PCR+ and CYT+PCR+ patients. PCR positivity also predicted a higher likelihood of disease recurrence after resection. An R0 resection was performed in 85 LAP− patients (54%): only 1 (1%) was CYT+, while 13 (15%) were PCR+. Of this group, PCR+ demonstrated a worse survival than PCR− patients (P = 0.02). Further analysis showed that, in R0 resection, stage III/IV, CYT− subgroup, PCR+ was associated with a trend towards worse survival (P = 0.09) compared to PCR− patients.
Conclusion
RT-PCR for CEA increases the detection of subclinical peritoneal disease and is more sensitive than cytology. Predictors of positive PCR included advanced-stage disease, vascular invasion, and perineural invasion. PCR positivity was associated with increased disease recurrence and decreased survival. Further follow-up is required to determine if PCR positivity alone is an independent predictor of poor survival in gastric cancer.



Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr
American Cancer Society.: Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society, 2010
Kim SJ, Kim HH, Kim YH, et al. Peritoneal metastasis: detection with 16− or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology, 2009; 253(2): 407–15.
Muntean V, Mihailov A, Iancu C, et al. Staging laparoscopy in gastric cancer. Accuracy and impact on therapy. Journal of Gastrointestinal and Liver Diseases, 2009; 18(2): 189–95.
Bentrem D, Wilton A, Mazumdar M, et al. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing curative resection. Annals of Surgical Oncology, 2004; 12(5): 1–7
La Torre M, Giovagnoli MR, Sforza N, et al. Peritoneal wash cytology in gastric carcinoma. Prognostic significance and therapeutic consequences. European Journal of Surgical Oncology, 2010; Oct; 36(10): 982–6.
Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC) Cancer Staging Manual (7th ed.). Chicago: Springer Inc., 2010.
Nakajiama T, Hirashima S, Hirata M, et al. Prognostic and therapeutic values of peritoneal cytology in gastric cancer. Acta Cytol, 1978; 22: 225–229.
Kodera Y, Yamamura Y, Shimizu Y, et al. Peritoneal Washing Cytology: Prognostic Value of Positive Findings in Patients with Gastric Carcinoma Undergoing a Potentially Curative Resection. Journal of Surgical Oncology, 1999; 72: 60–65.
Wilkiemeyer MB, Bieligk SC, Ashfaq R, et al. Laparoscopy alone is superior to peritoneal cytology in staging gastric and esophageal carcinoma. Surgical Endoscopy, 2004; 18(5): 852–856.
Lorenzen S, Panzram B, Rosenberg R, et al. Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Annals of Surgical Oncology, 2010; 17(10): 2733–9.
Mezhir JJ, Shah MA, Jacks LM, et al. Positive Peritoneal Cytology in Patients with Gastric Cancer: Natural History and Outcome of 291 Patients. Annals of Surgical Oncology, 2010; 17(12): 3173–80.
Moore Dalal K, Woo Y, Kelly K, et al. Detection of micrometastases in peritoneal washings of gastric cancer patients by the reverse transcriptase polymerase chain reaction. Gastric Cancer, 2008; 11:206–213.
Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Annals of Surgery, 2002; 235: 499–506.
Katsuragi K, Yashiro M, Sawada T, et al. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. British Journal of Cancer, 2007; 97: 550–556.
Yonemura Y, Endou Y, Fujimura T, et al. Diagnostic value of preoperative RT-PCR-based screening method to detect carcinoembryonic antigen-expressing free cancer cells in the peritoneal cavity from patients with gastric cancer. ANZ J Surgery, 2001; 71: 521–8.
Fujiwara Y, Doki Y, Taniguchi H, et al. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer, 2007; 10: 197–204.
Nath J, Moorthy K, Taniere P, et al. Peritoneal lavage cytology in patients with oesophagogastric adenocarcinoma. British Journal of Surgery, 2008; 95: 721–726.
Hur H, Lee HH, Jung H, et al. Predicting Factors of Unexplained Peritoneal Seeding in Locally Advanced Gastric Cancer: Indications for Staging Laparoscopy. Journal of Surgical Oncology, 2010; 102: 753–757.
Song KY, Kim JJ, Kim SN, et al. Staging Laparoscopy for Advanced Gastric Cancer: Is It also Useful for the Group Which Has an Aggressive Surgical Strategy? World Journal of Surgery, 2007; 31: 1228–1233.
Nicholl MB, Elashoff D, Takeuchi H, et al. Molecular upstaging based on paraffin-embedded sentinel lymph nodes: ten-year follow-up confirms prognostic utility in melanoma patients. Annals of Surgery, 2011; 253(1): 116−22.
Kelly KJ, Wong J, Gladdy R, et al. Prognostic Impact of RT-PCR-Based Detection of Peritoneal Micrometastases in Patients with Pancreatic Cancer Undergoing Curative Resection. Annals of Surgical Oncology, 2009; 16: 3333–3339.
Lloyd JM, McIver CM, Stephenson SA, et al. Identification of Early-Stage Colorectal Cancer Patients at Risk of Relapse Post-Resection by Immunobead Reverse Transcription-PCR Analysis of Peritoneal Lavage Fluid for Malignant Cells. Clinical Cancer Research, 2006; 12: 417–423.
Oyama K, Terashima M, Takagane A, et al. Prognostic significance of peritoneal minimal residual disease in gastric cancer detected by reverse transcription-polymerase chain reaction. British Journal of Surgery, 2004; 91(4): 435–43.
Wang JY, Lin SR, Lu CY, et al. Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Letters, 2005; 223(1): 129–35.
Kodera Y, Nakanishi H, Ito S, et al. Prognostic Significance of Intraperitoneal Cancer Cells in Gastric Carcinoma: Analysis of Real Time Reverse Transcriptase-Polymerase Chain Reaction after 5 Years of Followup. Journal of the American College of Surgeons, 2006; 202(2): 231–236.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. R. Daniel Beauchamp (Nashville, TN, USA): One limitation of peritoneal cytology for staging of gastric cancer is that is has a relatively low sensitivity, and may be negative, even in the presence of visible peritoneal disease. This paper by Joyce Wong, Dan Coit, and colleagues from Memorial Sloan–Kettering Cancer Center examines the use of quantitative real-time RT-PCR analysis of the tumor marker carcinoembryonic antigen (CEA) as a more sensitive method to detect evidence of occult metastatic spread of within the peritoneal cavity in a group of patients with gastric adenocarcinoma. In addition, the presence of PCR positivity was associated with significantly worse survival as compared with PCR-negative patients, although PCR-positive status did not achieve statistical significance in cytologically negative patients. Interestingly, 39% of patients who had laparoscopically detectable peritoneal disease were cytologically negative and, of these, only 47% were PCR positive. Thus, my first two questions are the following: Did these PCR-negative tumors express CEA, and is there perhaps a more sensitive and specific gastric cancer cell biomarker or set of biomarkers that may be used? Secondly, since the peritoneal washings were done by laparoscopy, using this information for decisions to proceed with resection in the absence of visible implants would require awaiting these results and a second procedure. Have the authors begun to use this approach for decisions regarding resection, and can it be done with peritoneal lavage under local anesthesia?
Closing Discussant
Dr. Joyce Wong: Thank you for the discussion of our paper. Addressing your first question, we did also note that PCR for CEA was not positive in all or the vast majority of patients with positive cytology. While we do not have all the pathology data pertaining to CEA specifically, we presume that not all gastric cancers are CEA-expressing. We did examine a number of biomarkers prior to initiating this study, including CK20 and several of the MUC genes; however, CEA was the single most sensitive marker. We chose to independently examine RT-PCR for CEA, although it would be useful in the future to determine whether a panel of biomarkers may enhance disease detection. As to your second point, we performed staging laparoscopy as a separate procedure so that cytology results were available at the time of resection. Our institution considers patients with positive cytology as unresectable and will stratify those patients towards chemotherapy. Laparoscopy was performed with minimal morbidity. There has been some discussion revolving around how to perform peritoneal lavage without general anesthesia, although we currently do not routinely perform any other approach for obtaining peritoneal lavage.
Rights and permissions
About this article
Cite this article
Wong, J., Kelly, K.J., Mittra, A. et al. RT-PCR Increases Detection of Submicroscopic Peritoneal Metastases in Gastric Cancer and Has Prognostic Significance. J Gastrointest Surg 16, 889–896 (2012). https://doi.org/10.1007/s11605-012-1845-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-012-1845-2