Abstract
Introduction
Although 5-year survival approaches 55% following resection of colorectal liver metastasis, most patients develop recurrent disease that is often isolated to the liver. Although repeat curative intent surgery (CIS) is increasingly performed for recurrent colorectal liver metastasis, only small series have been reported. We sought to determine safety and efficacy of repeat CIS for recurrent colorectal liver metastasis as well as determine factors predictive of survival in a large multicenter cohort of patients.
Methods
Between 1982 and 2008, 1,706 patients who underwent CIS—defined as curative intent hepatic resection/radiofrequency ablation (RFA)—for colorectal liver metastasis were identified from an international multi-institutional database. Two hundred forty-six (14.4%) patients underwent 301 repeat CIS. Data on clinico-pathologic factors, morbidity, and mortality were collected and analyzed.
Results
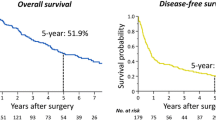
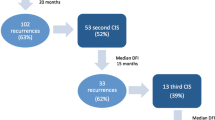
Following initial CIS, 645 (37.8%) patients had recurrence within the liver. Of these, 246 patients underwent repeat CIS for recurrent disease. The majority had hepatic resection alone as initial therapy (n = 219; 89.0%). A subset of patients underwent third (n = 46) or fourth (n = 9) repeat CIS. Mean interval between surgeries was similar (first → second, 19.1 months; second → third, 21.5 months; third → fourth, 11.3 months; P = 0.20). Extent of hepatic resection decreased with subsequent CIS (≥hemihepatectomy: first CIS, 30.9% versus second CIS, 21.1% versus third/fourth CIS, 16.4%; P = 0.004). RFA was utilized in one quarter of patients undergoing repeat CIS (second CIS, 21.1% versus third/fourth CIS, 25.5%). Mortality and morbidity were similar following second, third, and fourth CIS, respectively (all P > 0.05). Five-year survival was 47.1%, 32.6%, and 23.8% following the first, second, and third CIS, respectively. Presence of extra-hepatic disease was predictive of worse survival (HR = 2.26, P = 0.01).
Conclusion
Repeat CIS for recurrent colorectal liver metastasis can be performed with low morbidity and near-zero mortality. Patients with no extra-hepatic disease are best candidates for repeat CIS. In these patients, repeat CIS can offer the chance of long-term survival.



Similar content being viewed by others
References
Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer 2006;107(8):1711–1742.
Rudy DR, Zdon MJ. Update on colorectal cancer. Am Fam Physician 2000;61(6):1759–1770. 1773–1774.
Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin 2005;55(1):10–30.
Steele G Jr., Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg 1989;210(2):127–138.
Cady B, Monson DO, Swinton NW. Survival of patients after colonic resection for carcinoma with simultaneous liver metastases. Surg Gynecol Obstet 1970;131(4):697–700.
Blumgart LH, Allison DJ. Resection and embolization in the management of secondary hepatic tumors. World J Surg 1982;6(1):32–45.
Jatzko G, Wette V, Muller M, et al. Simultaneous resection of colorectal carcinoma and synchronous liver metastases in a district hospital. Int J Colorectal Dis 1991;6(2):111–114.
Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg 1986;73(9):732–735.
Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995;19(1):59–71.
Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am 2003;12(1):165–192. xi.
Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 1991;110(1):13–29.
Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008;13(1):51–64.
Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 2007;11(8):1057–1077.
Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239(6):818–825. discussion 825–827.
Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77(11):1241–1246.
Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235(6):759–766.
Hughes KS, Rosenstein RB, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis Colon Rectum 1988;31(1):1–4.
Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230(3):309–318. discussion 318–321.
Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984;119(6):647–651.
Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery 1994;116(4):703–710. discussion 710–711.
Jenkins LT, Millikan KW, Bines SD, et al. Hepatic resection for metastatic colorectal cancer. Am Surg 1997;63(7):605–610.
Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg 1997;132(5):505–510. discussion 511.
Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241(5):715–722. discussion 722–724.
Nordlinger B, Quilichini MA, Parc R, et al. Hepatic resection for colorectal liver metastases. Influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg 1987;205(3):256–263.
Bozzetti F, Doci R, Bignami P, et al. Patterns of failure following surgical resection of colorectal cancer liver metastases. Rationale for a multimodal approach. Ann Surg 1987;205(3):264–270.
Ekberg H, Tranberg KG, Andersson R, et al. Pattern of recurrence in liver resection for colorectal secondaries. World J Surg 1987;11(4):541–547.
Adam R, Bismuth H, Castaing D, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg 1997;225(1):51–60. discussion 60–62.
de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastases: an international multi-institutional analysis of 1669 patients. Ann Surg 2009;250(3):440–448.
Lange JF, Leese T, Castaing D, Bismuth H. Repeat hepatectomy for recurrent malignant tumors of the liver. Surg Gynecol Obstet 1989;169(2):119–126.
Pinson CW, Wright JK, Chapman WC, et al. Repeat hepatic surgery for colorectal cancer metastasis to the liver. Ann Surg 1996;223(6):765–773. discussion 773–776.
Nishio H, Hamady ZZ, Malik HZ, et al. Outcome following repeat liver resection for colorectal liver metastases. Eur J Surg Oncol 2007;33(6):729–734.
Yamamoto J, Kosuge T, Shimada K, et al. Repeat liver resection for recurrent colorectal liver metastases. Am J Surg 1999;178(4):275–281.
Petrowsky H, Gonen M, Jarnagin W, et al. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi–institutional analysis. Ann Surg 2002;235(6):863–871.
Sa Cunha A, Laurent C, Rault A, et al. A second liver resection due to recurrent colorectal liver metastases. Arch Surg 2007;142(12):1144–1149. discussion 1150.
Nordlinger B, Vaillant JC, Guiguet M, et al. Survival benefit of repeat liver resections for recurrent colorectal metastases: 143 cases. Association Francaise de Chirurgie. J Clin Oncol 1994;12(7):1491–1496.
Tuttle TM, Curley SA, Roh MS. Repeat hepatic resection as effective treatment of recurrent colorectal liver metastases. Ann Surg Oncol 1997;4(2):125–130.
Stone MD, Cady B, Jenkins RL, et al. Surgical therapy for recurrent liver metastases from colorectal cancer. Arch Surg 1990;125(6):718–721. discussion 722.
Vaillant JC, Balladur P, Nordlinger B, et al. Repeat liver resection for recurrent colorectal metastases. Br J Surg 1993;80(3):340–344.
Fong Y, Blumgart LH, Cohen A, et al. Repeat hepatic resections for metastatic colorectal cancer. Ann Surg 1994;220(5):657–662.
Nagakura S, Shirai Y, Suda T, Hatakeyama K. Multiple repeat resections of intra- and extrahepatic recurrences in patients undergoing initial hepatectomy for colorectal carcinoma metastases. World J Surg 2002;26(2):141–147.
Brachet D, Lermite E, Rouquette A, et al. Prognostic factors of survival in repeat liver resection for recurrent colorectal metastases: review of sixty-two cases treated at a single institution. Dis Colon Rectum 2009;52(3):475–483.
Clavien PA, Emond J, Vauthey JN, et al. Protection of the liver during hepatic surgery. J Gastrointest Surg 2004;8(3):313–327.
Strasberg SM. For the International Hepato-Pancreato-Biliary Association Terminology Committee Survey. The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB 2000;2(3):333–339.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Am Stat Assoc J 1958;53:457–480.
Fernandez-Trigo V, Shamsa F, Sugarbaker PH. Repeat liver resections from colorectal metastasis. Repeat Hepatic Metastases Registry. Surgery 1995;117(3):296–304.
Aramaki M, Kawano K, Kai T, et al. Postoperative complications of repeat hepatectomy for liver metastasis from colorectal carcinoma. Hepatogastroenterology 2000;47(32):478–480.
Elias D, Lasser P, Hoang JM, et al. Repeat hepatectomy for cancer. Br J Surg 1993;80(12):1557–1562.
Adam R, Pascal G, Azoulay D, et al. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg 2003;238(6):871–883. discussion 883–884.
Ishiguro S, Akasu T, Fujimoto Y, et al. Second hepatectomy for recurrent colorectal liver metastasis: analysis of preoperative prognostic factors. Ann Surg Oncol 2006;13(12):1579–1587.
Pawlik TM, Izzo F, Cohen DS, et al. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 2003;10(9):1059–1069.
Elias D, De Baere T, Smayra T, et al. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg 2002;89(6):752–756.
Tsai S, Pawlik TM. Outcomes of ablation versus resection for colorectal metastases: are we comparing apples to oranges? Ann Surg Oncol 2009;16(9):2422–2428.
Gleisner AL, Choti MA, Assumpcao L, et al. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg 2008;143(12):1204–1212.
Asiyanbola B, Chang D, Gleisner AL, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg 2008;12(5):842–851.
Muratore A, Polastri R, Bouzari H, et al. Repeat hepatectomy for colorectal liver metastases: A worthwhile operation? J Surg Oncol 2001;76(2):127–132.
Pessaux P, Lermite E, Brehant O, et al. Repeat hepatectomy for recurrent colorectal liver metastases. J Surg Oncol 2006;93(1):1–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mechteld C. De Jong, presenter (medical student from the Netherlands)
Discussant
Dr. Sean Mulvihill (Salt Lake City, UT): For those of you who missed it, Miss DeJong is a medical student. Very nicely done. I hope this experience encourages you to seek a career in surgery.
This is the largest reported series of repeat, curative intent liver surgery for colorectal metastases to the liver. But these operations were uncommon. By my calculation, only about two operations were done per center, per year, given that there were five centers over 20-some years in this study.
In our own hospital it seems like this scenario is increasing in frequency, and I wonder if that’s been your experience? I think we would agree that these could be technically difficult operations in terms of dissecting the liver off the diaphragm, stomach and colon, particularly at the site of the prior resection. And that makes me wonder whether we should be considering use of some anti-adhesion barrier, such as Seprafilm, at the time of the primary liver resection.
Staging is critically important to ensure identification of all disease, and I’m sure that over the 20-odd years in this study the methods of staging changed. And I wonder if could you tell us what your current standard for axial imaging of the chest and abdomen is, and your current use of PET.
I was surprised that chemotherapy was only used in about two-thirds of the patients in this series. And I think, from what we heard today, there is some difference of opinion about the use of chemotherapy. We would favor it on both a neoadjuvant and postoperative adjuvant basis for liver resection for colorectal metastasis. Please tell us what your current standard for the use of chemotherapy is.
Closing discussant
Mechteld C. De Jong: Thank you for your questions. Because of my English I will ask if Dr. Pawlik can assist in responding to your questions.
Closing discussant
Dr. Timothy M. Pawlik (Johns Hopkins, Baltimore, Maryland): Thank you very much for reviewing our paper.
With regard to your first question, there was a trend over time whereby repeat hepatectomies were more frequently performed over the last decade. I think repeat hepatectomy may be more frequently used because liver resection is now associated with a much lower operative morbidity and mortality and we are armed with more effective, systemic chemotherapy to complement surgery. However, you are correct in that the study did occur over a long time period and this should be considered when interpreting the conclusions.
We did not investigate the use of Seprafilm or other anti-adhesive agents. I personally do not routinely use Seprafilm at the time of initial hepatic resection. The field is also quickly changing and perhaps as more and more initial hepatectomies are performed either laparoscopically or with the robot, we may find that repeat hepatectomies may become an easier operation.
Your third question related to the use of cross-sectional imaging. Most centers used CT scans. At Johns Hopkins, we generally obtain both a CT scan as well as a pre-operative PET scan. However, many of the centers—including those in Europe—did not routinely obtain a pre-operative PET scan.
In general, we use chemotherapy in the adjuvant setting for patients who have resectable liver disease and use it preoperatively for those patients with borderline or unresectable disease in the hopes of converting them to surgical resection. For those patients who present with synchronous disease with an asymptomatic primary colorectal cancer in place, we strongly favor treating this group of patients with preoperative chemotherapy. Also, for those patients who have both intra- and extra-extra-hepatic disease (who constituted about 20% of the current study) we also strongly favor preoperative chemotherapy. The use of chemotherapy in the setting of repeat hepatectomy is more complicated and may depend not only on the interval from their recurrence, but also on how long the patient has been chemo naive, what chemotherapy they may have received in the past, etc. The chemotherapy question in these patients needs to be addressed on an individual case-by-case basis.
Discussant
Dr. Kaye M. Reid Lombardo (Mayo Clinic , Rochester, MN): In the group of patients who had extra-hepatic disease, what was the extent of their disease? Did they have multiple sites involved? And/or whether or not they were surgically treated as well?
Closing discussant
Mechteld C. De Jong: Thank you for your question. The majority of patients who had extra-hepatic disease had a solitary, lung metastasis. Only patients who had limited, extra-hepatic disease were included in our study. In general, patients with intra- and extra-hepatic disease were first treated with systemic chemotherapy and had a demonstrable response or stable disease following chemotherapy. Only patients in whom both the intra- and extra-hepatic disease could be resected with an R0 margin were included in the study.
Support: Dr. Pawlik is supported by grant number 1KL2RR025006-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Rights and permissions
About this article
Cite this article
de Jong, M.C., Mayo, S.C., Pulitano, C. et al. Repeat Curative Intent Liver Surgery is Safe and Effective for Recurrent Colorectal Liver Metastasis: Results from an International Multi-institutional Analysis. J Gastrointest Surg 13, 2141–2151 (2009). https://doi.org/10.1007/s11605-009-1050-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-1050-0