Abstract
Introduction
Survival after surgery of pancreatic cancer is still poor, even after curative resection. Some prognostic factors like the status of the resection margin, lymph node (LN) status, or tumor grading have been identified. However, only few data have been published regarding the prognostic influence of the LN ratio (number of LN involved to number of examined LN). We, therefore, evaluated potential prognostic factors in 182 patients after resection of pancreatic cancer including assessment of LN ratio.
Methods
Since 1994, 204 patients underwent pancreatic resection for ductal pancreatic adenocarcinoma. Survival was evaluated in 182 patients with complete follow-up evaluations. Of those 182 patients, 88% had cancer of the pancreatic head, 5% of the body, and 7% of the pancreatic tail. Patients underwent pancreatoduodenectomy (85%), distal resection (12%), or total pancreatectomy (3%). Survival was analyzed by the Kaplan–Meier and Cox methods.
Results
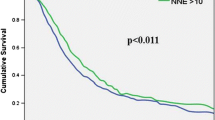
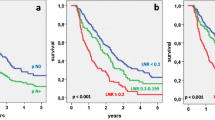
In all 204 resected patients, operative mortality was 3.9% (n = 8). In the 182 patients with follow-up, 70% had free resection margins, 62% had G1- or G2-classified tumors, and 70% positive LN. Median tumor size was 30 (7–80) mm. The median number of examined LN was 16 and median number of involved LN 1 (range 0–22). Median LN ratio was 0.1 (0–0.79). Cumulative 5-year survival (5-year SV) in all patients was 15%. In univariate analysis, a LN ratio ≥ 0.2 (5-year SV 6% vs. 19% with LN ratio < 0.2; p = 0.003), LN ratio ≥ 0.3 (5-year SV 0% vs. 18% with LN ratio < 0.3; p < 0.001), a positive resection margin (p < 0.01) and poor differentiation (G3/G4; p < 0.03) were associated with poorer survival. In multivariate analysis, a LN ratio ≥ 0.2 (p < 0.02; relative risk RR 1.6), LN ratio ≥ 0.3 (p < 0.001; RR 2.2), positive margins (p < 0.02; RR 1.7), and poor differentiation (p < 0.03; RR 1.5) were independent factors predicting a poorer outcome. The conventional nodal status or the number of examined nodes (in all patients and in the subgroups of node positive or negative patients) had no significant influence on survival. Patients with one metastatic LN had the same outcome as patients with negative nodes, but prognosis decreased significantly in patients with two or more LN involved.
Conclusions
Not the lymph node involvement per se but especially the LN ratio is an independent prognostic factor after resection of pancreatic cancers. In our series, the LN ratio was even the strongest predictor of survival. The routine estimation of the LN ratio may be helpful not only for the individual prediction of prognosis but also for the indication of adjuvant therapy and herein related outcome and therapy studies.





Similar content being viewed by others
References
House MG, Gonen M, Jarnagin WR, D'Angelica M, Dematteo RP, Fong Y, Brennan MF, Allen PJ. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. doi:10.1007/s11605-007-0243-7.
Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi:10.1016/j.gassur.2006.08.018.
Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. doi:10.1007/s00268-002-6659-z.
Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA, Evans DB. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi:10.1016/j.gassur.2004.09.046.
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366. doi:10.1097/00000658-200209000-00012.
Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, Abrams RA, Laheru D, Hruban RH, Yeo CJ. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma-part 3: update on 5-year survival. J Gastrointest Surg. 2005;9:1191–1206. doi:10.1016/j.gassur.2005.08.034.
Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, Kloppel G, Dhaene K, Michelassi F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508–517. doi:10.1097/00000658-199810000-00007.
Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Buchler MW. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi:10.1016/S0140-6736(01)06651-X.
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi:10.1001/jama.297.3.267.
Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi:10.1097/00000658-200301000-00011.
Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, Wolff RA, Abbruzzese JL, Janjan NA, Crane CH, Vauthey JN, Lee JE, Pisters PW, Evans DB. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–132. doi:10.1007/s10434-001-0123-4.
Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi:10.1097/01.sla.0000133125.85489.07.
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi:10.1016/S1091-255X(00)80105-5.
Riediger H, Makowiec F, Fischer E, Adam U, Hopt UT. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg. 2006;10:1106–1115. doi:10.1016/j.gassur.2006.04.002.
Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, Wolfgang C, Hruban RH, Schulick RD, Yeo CJ, Choti MA. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi:10.1016/j.surg.2006.12.013.
Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70:235–240.
Sierzega M, Popiela T, Kulig J, Nowak K. The ratio of metastatic/resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic head cancer. Pancreas. 2006;33:240–245. doi:10.1097/01.mpa.0000235306.96486.2a.
Adam U, Makowiec F, Riediger H, Schareck WD, Benz S, Hopt UT. Risk factors for complications after pancreatic head resection. Am J Surg. 2004;187:201–208. doi:10.1016/j.amjsurg.2003.11.004.
Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, Wolff RA, Abbruzzese JL, Janjan NA, Crane CH, Vauthey JN, Lee JE, Pisters PW, Evans DB. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–132. doi:10.1007/s10434-001-0123-4.
Ishikawa O, Ohhigashi H, Sasaki Y, Kabuto T, Fukuda I, Furukawa H, Imaoka S, Iwanaga T. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann Surg. 1988;208:215–220. doi:10.1097/00000658-198808000-00014.
Manabe T, Ohshio G, Baba N, Miyashita T, Asano N, Tamura K, Yamaki K, Nonaka A, Tobe T. Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer. 1989;64:1132–1137. doi:10.1002/1097-0142(19890901)64:5<1132::AID-CNCR2820640528>3.0.CO;2-V.
Bogoevski D, Onken F, Koenig A, Kaifi JT, Schurr P, Sauter G, Izbicki JR, Yekebas EF. Is it time for a new TNM classification in esophageal carcinoma? Ann Surg. 2008;247:633–641. doi:10.1097/SLA.0b013e3181656d07.
Wijnhoven BP, Tran KT, Esterman A, Watson DI, Tilanus HW. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg. 2007;245:717–725. doi:10.1097/01.sla.0000251703.35919.02.
Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–784.
Sierra A, Regueira FM, Hernandez-Lizoain JL, Pardo F, Martinez-Gonzalez MA, Cienfuegos J. Role of the extended lymphadenectomy in gastric cancer surgery: experience in a single institution. Ann Surg Oncol. 2003;10:219–226. doi:10.1245/ASO.2003.07.009.
Rosenberg R, Friederichs J, Schuster T, Gertler R, Maak M, Becker K, Grebner A, Ulm K, Hofler H, Nekarda H, Siewert JR. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann Surg. 2008;248:968–978. doi:10.1097/SLA.0b013e318190eddc.
Bogoevski D, Yekebas EF, Schurr P, Kaifi JT, Kutup A, Erbersdobler A, Pantel K, Izbicki JR. Mode of spread in the early phase of lymphatic metastasis in pancreatic ductal adenocarcinoma: prognostic significance of nodal microinvolvement. Ann Surg. 2004;240:993–1000. doi:10.1097/01.sla.0000145922.25106.e3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 49th Annual Meeting of the Society for Surgery of the Alimentary Tract, May 2008 in San Diego and at the Annual Meeting of the German Cancer Society, February 2008 in Berlin, Germany
Rights and permissions
About this article
Cite this article
Riediger, H., Keck, T., Wellner, U. et al. The Lymph Node Ratio is the Strongest Prognostic Factor after Resection of Pancreatic Cancer. J Gastrointest Surg 13, 1337–1344 (2009). https://doi.org/10.1007/s11605-009-0919-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-0919-2