Abstract
Objective
Pancreatic cancer patients with positive (+) peritoneal cytology have a prognosis similar to stage IV patients. We studied the ability of quantitative real time-polymerase chain reaction (RT-PCR) to detect micrometastases in patients undergoing staging laparoscopy.
Methods
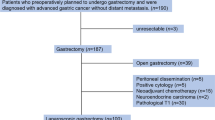
Peritoneal washes were obtained prospectively from 35 consecutive patients with pancreatic adenocarcinoma undergoing staging laparoscopy and 16 patients undergoing laparoscopy for benign disease. Each sample was assessed by cytologic examination and RT-PCR analysis for tumor markers: CEA, CK7, Kras2, and MUC1. Markers and their combinations were evaluated on the basis of their deviance from the ideal marker.
Results
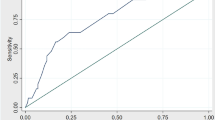
Pathologic stages for pancreatic cancer patients were: 1A-1 (3%), IB-1 (3%), IIA-5 (15%), IIB-13 (38%), III-5 (15%), IV-9 (26%). Eight patients were cytology (+) and stages IIA-1, IIB-2, IV-5. Twenty-five patients were RT-PCR (+). The optimal threshold for cycle amplification was 35 based on a receiver operating characteristic curve. CEA had the best profile of sensitivity, specificity, PPV, NPV, and the smallest deviance.
Conclusion
RT-PCR using a panel of tumor markers, including CEA, was comparable in sensitivity, specificity, PPV, and NPV to cytology. RT-PCR could represent a more sensitive method for detection of subclinical peritoneal tumor dissemination; this may be useful in patient selection for operative management and clinical trials.


Similar content being viewed by others
References
Conlon KC, Klimstra D, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996;223:273–279.
Henne-Bruns D, Vogel I, Luttges J, Kloppel G, Kremer B. Ductal adenocarcinoma of the pancreas head: survival after regional versus extended lymphadenectomy. Hepatogastroenterology 1998;45:855–866.
Warshaw AL. Implications of malignant-cell DNA content for treatment of patients with pancreatic cancer. Ann Surg 1991;214:645–647.
Merchant NB, Conlon K, Saigo P, Dougherty E, Brennan MF. Positive peritoneal cytology predicts unresectability of pancreatic adenocarcinoma. J Am Coll Surg 1999;188:421–426.
Ferrone CR, Haas B, Tang L, Coit DG, Fong Y, Brennan MF, Allen PJ. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg 2006;10:1347–53.
Vogel I, Kruger U, Marxsen J, Soeth E, Kalthoff H, Henne-Bruns D, Kremer B, Juhl H. Disseminated tumor cells in pancreatic cancer patients detected by immunocytology: a new prognostic factor. Clin Cancer Res 1991;5:593–599.
Kodera Y, Nakanishi H, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T, Tatematsu M. Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptase-polymerase chain reaction and cytology. Int J Cancer 1998;79:429–433.
Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T, Tatematsu M. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg 2002;235:499–506.
Kodera Y, Nakanishi H, Ito S, Yamamura Y, Fujiwara M, Koike M, Hibi K, Ito K, Tatematsu M, Nakao A. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer 2005;8:142–148.
Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol 1999;72:60–64.
Yamashita K, KidaY, Shinoda H, Kida M, Okayasu I. K-ras point mutations in the supernatants of pancreatic juice and bile are reliable for diagnosis of pancreas and biliary tract carcinomas complementary to cytologic examination. Jpn J Cancer Res 1999;90:240–248.
Hornick JL, Lauwers G, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol 2005;29:381–389.
Chu PG, Schwarz R, Lau SK, Yen Y, Weiss LM. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol 2005;29:359–367.
Goldstein NS, Bassi D. Cytokeratins 7, 17, and 20 reactivity in pancreatic and ampulla of Vater adenocarcinomas. Percentage of positivity and distribution is affected by the cut-point threshold. Am J Clin Pathol 2001;115:695–702.
Sipos B, Moser S, Kalthoff H, Torok V, Lohr M, Kloppel G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Arch 2003;442(5):444–445.
Zweigh MH, Campbell G. Receiver-operating characteristics (ROC) plots, a fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561–577.
Acknowledgments
We would like to acknowledge members of the Fong Laboratory, Yun Shin Chun, MD, and David Eisenberg, MD, as well as research study assistants Judy Fong and Maria Janakos.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCUSSION
Keith D. Lillemoe, M.D. (Indianapolis, IN): Kim, that was a very nice presentation. Seeing you up there in uniform reminds me that I think both myself and immediate Past President Dr. Bass had our first SSAT presentations in uniform too. So I think your future looks bright in this organization, as we all knew it would be.
I have a few questions. I guess the first thing is, really, what value is this? If I am not correct, you do your laparoscopic staging at the time that you plan to explore the patients for resection. In other words, you are really not using this information at all for clinical decision-making. So I guess the first question is, when are you going to believe your cytology data to start doing it as a planned procedure, a day, a week or whatever it takes, before you can plan exploration for resection? A lot of groups talk about the value of peritoneal cytology, but when the rubber hits the road, in their clinical practice, they do not really practice the use of it that much.
Let us just assume that this analysis was of value. What is the cost associated with this analysis? In the end, we will be adding not only this, but a separate laparoscopic procedure with general anesthesia, some time in the hospital, or at least in the recovery room. Is this really going to have a cost/benefit ratio in helping the small number of patients where it is going to be of benefit?
Obviously you alluded to it, but we obviously want to see a second paper showing the follow-up of these people with a “false positive” RT-PCR positivity to see if indeed their survival is affected.
Finally, where I think this analysis might be of value are those patients who we see in follow-up after pancreatic resection who develop ascites. Many times we do not know the cause of that ascites. If we could tap the ascites and find out definitely if they have malignant disease, it may help us better direct their therapy.
It is a beautiful study. I am not sure if it is quite ready for prime time application to all those people who are doing pancreatic surgery, but I think it adds to our knowledge of the disease. Nice presentation.
Kimberly M. Dalal, M.D. (Travis AFB, CA): Thank you for your comments and for your support. When will we believe the data and actually apply them to our patients? Well, that is probably not for at least 5 years. Now that this pilot study has established the feasibility and optimized the primers and parameters for RT-PCR, Drs. Fong and Coit and a couple of current surgical oncology fellows have taken on a prospective study looking at 200 patients with pancreatic adenocarcinoma. To accrue 200 patients and to obtain 2 years of follow-up will take about 5 years. I agree; actually using this information to make treatment decisions is not something we can do immediately.
With regard to your second question about the cost benefit of RT-PCR, I will say that to perform laparoscopy under general anesthesia at a separate setting and to process the peritoneal wash samples for RNA isolation and RT-PCR for the panel of tumor markers will cost several hundred to a thousand dollars. However, if we could identify patients who would not benefit from surgery, this would provide a tremendous benefit that may outweigh that initial cost by preventing the potential morbidity that can be associated with pancreatic resections, decrease in quality of life, and additional costs that may result from an operation that ultimately would not lead to a curative resection. This procedure may, in fact, come out to have a definite cost benefit. But again, we will not know this for at least 5 years.
Craig P. Fischer, M.D. (Houston, TX): How about the use for malignant ascites, do you think that that has some utility?
Dr. Dalal: I think it does. We have not pursued that ourselves, but I think that it is definitely something to consider investigating in future studies.
Sources of support: none
Rights and permissions
About this article
Cite this article
Dalal, K.M., Woo, Y., Galanis, C. et al. Detection of Micrometastases in Peritoneal Washings of Pancreatic Cancer Patients by the Reverse Transcriptase Polymerase Chain Reaction. J Gastrointest Surg 11, 1598–1606 (2007). https://doi.org/10.1007/s11605-007-0283-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-007-0283-z