Summary
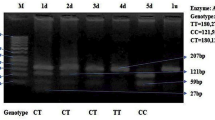
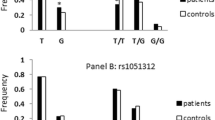
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by high heritability. Recently, autism, the most profound form of ASD, has been increasingly attributed to synaptic abnormalities. Postsynaptic density 95 (PSD95), encoding PSD protein-95, was found essential for synaptic formation, maturation and plasticity at a PSD of excitatory synapse. It is possibly a crucial candidate gene for the pathogenesis of ASD. To identify the relationship between the rs13331 of PSD95 gene and ASD, we performed a case-control study in 212 patients and 636 controls in a Chinese population by using a polymerase chain reaction-restriction fragment length polymerase (PCR-RFLP) assay. The results showed that in genetic analysis of the heterozygous model, an association between the T allele of the rs13331 and ASD was found in the dominant model (OR=1.709, 95% CI 1.227–2.382, P=0.002) and the additive model (OR=1.409, 95% CI=1.104–1.800, P=0.006). Our data indicate that the genetic mutation C>T at the rs13331 in the PSD95 gene is strikingly associated with an increased risk of ASD.
Similar content being viewed by others
References
Sandin S, Lichtenstein P, Kuja-Halkola R, et al. The familial risk of autism. JAMA, 2014,311(17):1770–1777
Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res, 2012,5(3):160–179
Rumsey JM, Rapoport JL, Sceery WR. Autistic children as adults: psychiatric, social, and behavioral outcomes. J AmAcad Child Psychiatry, 1985,24(4):465–473
Kao YC, Kramer JM, Liljenquist K, et al. Association between impairment, function, and daily life task management in children and adolescents with autism. Dev Med Child Neurol, 2015,57(1):68–74
Visser EM, Berger HJ, Van Schrojenstein LVH, et al. Cognitive shifting and externalising problem behaviour in intellectual disability and autism spectrum disorder. J Intellect Disabil Res, 2015,59(8):755–766
Baron-Cohen S. Social and pragmatic deficits in autism: cognitive or affective? J Autism Dev Disord, 1988,18(3): 379–402
Eicher JD, Gruen JR. Language impairment and dyslexia genes influence language skills in children with autism spectrum disorders. Autism Res, 2014,8(2):229–234
Helt M, Kelley E, Kinsbourne M, et al. Can children with autism recover? If so, how? Neuropsychol Rev, 2008,18(4):339–366
Cohen S, Conduit R, Lockley SW, et al. The relationship between sleep and behavior in autism spectrum disorder (ASD): a review. J Neurodev Disord, 2014,6(1):44
Ganz ML. The lifetime distribution of the incremental societal costs of autism. Arch Pediatr Adolesc Med, 2007,161(4):343–349
Ahmedani BK, Hock RM. Health care access and treatment for children with co-morbid autism and psychiatric conditions. Soc Psychiatry Psychiatr Epidemiol, 2012,47(11): 1807–1814
Montes G HJ. Child care problems and employment among families with preschool-aged children with autism in the United States. Pediatrics, 2008,122(1):202–208
Nordenbaek C, Jorgensen M, Kyvik KO, et al. A Danish population-based twin study on autism spectrum disorders. Eur Child Adolesc Psychiatry, 2014,23(1):35–43
Folstein S, Rutter M. Genetic influences and infantile autism. Nature, 1977,265(5596):726–728
Rosenberg RE, Law JK, Yenokyan G, et al. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch PediatrAdolesc Med, 2009,163(10):907–914
Lichtenstein P, Carlstrom E, Rastam M, et al. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry, 2010,167(11): 1357–1363
Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry, 2011,68(11):1095–1102
Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science, 2003,302(5646):826–830
van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep, 2010,10(3):207–214
Gai X, Xie HM, Perin JC, et al. Rare structural variation of synapse and neurotransmission genes in autism. Mol Psychiatry, 2012,17(4):402–411
Gylys KH, Fein JA, Yang F, et al. Synaptic changes in Alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol, 2004,165(5):1809–1817
Aarts M, Liu Y, Liu L, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science, 2002,298(5594):846–850
Cheng MC, Lu CL, Luu SU, et al. Genetic and functional analysis of the DLG4 gene encoding the post-synaptic density protein 95 in schizophrenia. PLoS One, 2010,5(12): e15107
Chen J, Yu S, Fu Y, et al. Synaptic proteins and receptors defects in autism spectrum disorders. Front Cell Neurosci, 2014,8:276
Taft CE, Turrigiano GG. PSD-95 promotes the stabilization of young synaptic contacts. Philos Trans R SocLond B BiolSci, 2014,369(1633):20130134
Migaud M, Charlesworth P, Dempster M, et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature, 1998, 396(6710):433–439
Leuba G, Walzer C, Vernay A, et al. Postsynaptic density protein PSD-95 expression in Alzheimer’s disease and okadaic acid induced neuritic retraction. Neurobiol Dis, 2008,30(3):408–419
Chiocchetti AG, Kopp M, Waltes R, et al. Variants of the CNTNAP2 5' promoter as risk factors for autism spectrum disorders: a genetic and functional approach. Mol Psychiatry, 2014,20(7):839–849
Arking DE, Cutler DJ, Brune CW, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet, 2008,82(1):160–164
Shao S, Xu S, Yang J, et al. A commonly carried genetic variant, rs9616915, in SHANK3 gene is associated with a reduced risk of autism spectrum disorder: replication in a Chinese population. MolBiol Rep, 2014,41(3):1591–1595
Chang SC, Pauls DL, Lange C, et al. Sex-specific association of a common variant of the XG gene with autism spectrum disorders. Am J Med Genet B Neuropsychiatr Genet, 2013,162B(7):742–750
Lord C, Cook EH, Leventhal BL, et al. Autism spectrum disorders. Neuron, 2000,28(2):355–363
Kennedy MB. Signal-processing machines at the postsynaptic density. Science, 2000,290(5492):750–754
Stephenson FA. Structure and trafficking of NMDA and GABAA receptors. BiochemSoc Trans, 2006,34(Pt 5):877–881
Uchino S, Wada H, Honda S, et al. Direct interaction of post-synaptic density-95/Dlg/ZO-1 domaincontainingsynaptic molecule Shank3 with GluR1alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor. J Neurochem, 2006,97(4):1203–1214
Zheng S, Gray EE, Chawla G, et al. PSD-95 is post-transcriptionally repressed during early neural development by PTBP1 and PTBP2. Nat Neurosci, 2012,15(3): 381–388, S1
Chen L, Chetkovich DM, Petralia RS, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature, 2000,408(6815):936–943
Stathakis DG, Hoover KB, You Z, et al. Human postsynaptic density-95 (PSD95): location of the gene (DLG4) and possible function in nonneural as well as in neural tissues. Genomics, 1997,44(1):71–82
He Z, Shao S, Zhou J, et al. Does long time spending on the electronic devices affect the reading abilities? A cross-sectional study among Chinese school-aged children. Res Dev Disabil, 2014,35(12):3645–3654
Sun Z, Zou L, Zhang J, et al. Prevalence and associated risk factors of dyslexic children in a middle-sized city of China: a cross-sectional study. PLoS One, 2013,8(2): e56688
Feyder M, Karlsson RM, Mathur P, et al. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams’ syndrome. Am J Psychiatry, 2010, 167(12):1508–1517
Author information
Authors and Affiliations
Corresponding author
Additional information
Both authors contributed equally to this work.
This work was supported by grants from the Fundamental Research Funds for the Central Universities (HUST No. 2015TS096), Hubei Province Health and Family Planning Scientific Research Project (No. WJ2015MB019), and a funding program of Science and Technology Projects of Shenzhen (No. JCYJ20150403142731429).
Rights and permissions
About this article
Cite this article
Wang, J., Li, L., Shao, Ss. et al. Association analysis of genetic variant of rs13331 in PSD95 gene with autism spectrum disorders: A case-control study in a Chinese population. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 36, 285–288 (2016). https://doi.org/10.1007/s11596-016-1581-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-016-1581-z