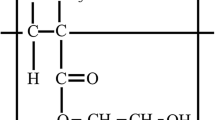
Abstract
The thin film of poly(methyl methacrylate) (PMMA) has been widely studied as host in a polymer electrolyte system due to its good mechanical stability towards lithium electrode. However, the brittle property of this film creates additional resistance for the ionic conduction. The addition of ionic liquid (IL) has been noticed to improve the brittleness of the film. In addition, its ionic conductivity can also be enhanced, but no free standing film can be obtained when higher amount of IL was added. Therefore, in this study, 1-methyl-3-pentamethyldisiloxymethylimidazolium bis(trifluoromethylsulfonyl)imide,[(SiOSi)C1C1im][NTf2], was incarcerated during free radical polymerization of MMA. Interestingly, this newly synthesized PMMA (PMMAIL) gives a flexible and transparent film with ionic conductivity of ∼10−7 S/cm at room temperature. The structural properties of this PMMAIL were further investigated using Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and optical microscope (OM).










Similar content being viewed by others
References
Appetecchi GB, Croce F, Scrosati B (1995) Kinetics and stability of the lithium electrode in poly (methylmethacrylate)-based gel electrolytes. Electrochim Acta 40(8):991–997
Latif FA (2006) Preparation and characterization of poly (methyl methacrylate)/50 % epoxidised natural rubber based solid electrolytes for lithium-ion secondary battery (Doctoral dissertation, Universiti Teknologi Malaysia).
Latif F, Aziz M, Katun N, Yahya MZA (2006) The role and impact of rubber in poly (methyl methacrylate)/lithium triflate electrolyte. J Power Sources 159(2):1401–1404
Rajendran S, Sivakumar M, Subadevi R (2004) Investigations on the effect of various plasticizers in PVA–PMMA solid polymer blend electrolytes. Mater Lett 58(5):641–649
Chew KW, Tan KW (2011) The effects of ceramic fillers on PMMA-based polymer electrolyte salted with lithium triflate, LiCF3SO3. Int J Electrochem Sci 6:5792–5801
Rajendran S, Mahendran O, Kannan R (2002) Ionic conductivity studies in composite solid polymer electrolytes based on methyl methacrylate. J Phys Chem Solids 63(2):303–307
Jiang J, Gao D, Li Z, Su G (2006) Gel polymer electrolytes prepared by in situ polymerization of vinyl monomers in room-temperature ionic liquids. React Funct Polym 66(10):1141–1148
Hawker CJ (1997) “Living” free radical polymerization: a unique technique for the preparation of controlled macromolecular architectures. Acc Chem Res 30(9):373–382
Rani MA (2012) Synthesis, characterization and physical-chemical properties of room temperature ionic liquids with a variety of cations, paired with bis (trifluoromethylsulfonyl) imide anion. doctoral dissertation, Imperial College, London
Hong K, Zhang H, Mays JW, Visser AE, Brazel CS, Holbrey JD, Rogers RD (2002) Conventional free radical polymerization in room temperature ionic liquids: a green approach to commodity polymers with practical advantages. Chem Commun 13:1368–1369
Ramesh S, Wen LC (2010) Investigation on the effects of addition of SiO2 nanoparticles on ionic conductivity, FTIR, and thermal properties of nanocomposite PMMA–LiCF3SO3–SiO2. Ionics 16(3):255–262
Chowdari BVR, Gopalakrishnan R, Tan KL (1990) ESCA studies of fast ion conducting glasses. Solid State Ionics 40:709–713
Deraman SK, Mohamed NS, Subban RHY (2014) Ionic liquid incorporated PVC based polymer electrolytes: electrical and dielectric properties. Sains Malaysiana 43(6):877–883
Ramesh S, Arof AK (2001) Ionic conductivity studies of plasticized poly (vinyl chloride) polymer electrolytes. Mater Sci Eng B 85(1):11–15
Shukla N, Thakur AK (2009) Role of salt concentration on conductivity optimization and structural phase separation in a solid polymer electrolyte based on PMMA-LiClO4. Ionics 15(3):357–367
Liew CW, Ramesh S, Durairaj R (2012) Impact of low viscosity ionic liquid on PMMA–PVC–LiTFSI polymer electrolytes based on AC-impedance, dielectric behavior, and HATR–FTIR characteristics. J Mater Res 27(23):2996–3004
Singh B, Sekhon SS (2005) Ion conducting behaviour of polymer electrolytes containing ionic liquids. Chem Phys Lett 414(1):34–39
Abidin SZZ, Yahya MZA, Hassan OH, Ali AMM (2014) Conduction mechanism of lithium bis (oxalato) borate–cellulose acetate polymer gel electrolytes. Ionics 20(12):1671–1680
Khiar ASA, Puteh R, Arof AK (2006) Conductivity studies of a chitosan-based polymer electrolyte. Phys B Condens Matter 373(1):23–27
Acknowledgments
The authors would like to express their gratitude to the Faculty of Applied Sciences, UiTM, i-MADE Lab and Institute of Science for their support in providing research facilities to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zailani, N.A.M., Latif, F.A., Ali, A.M.M. et al. Effect of ionic liquid incarceration during free radical polymerization of PMMA on its structural and electrical properties. Ionics 23, 295–301 (2017). https://doi.org/10.1007/s11581-016-1827-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1827-6