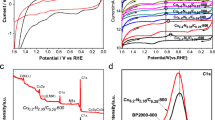
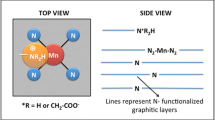
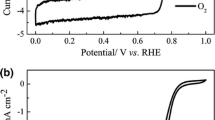
Abstract
Non-precious metal electrocatalysts obtained by pyrolysis of precursors of metal, nitrogen, and carbon (MNC) are viewed as an inexpensive replacement for platinum-based electrocatalysts for the oxygen reduction reaction (ORR) in fuel cells. The hypothesized ORR active site structure of typical MNC catalysts consists of a transition metal coordinated to the pyridinic/pyrollic type of nitrogen covalently attached to the edges of the graphitic crystallites. One of the drawbacks of all the reported procedures to synthesize these MNC electrocatalysts is the inability to control the formation of a specific active site structure suitable for ORR. Lack of clarity on the active site structure limits the researcher’s ability to design a synthesis methodology that maximizes the specific active site density. In this study, we have synthesized a Co(III) dimer ([Co2(OH)2(OOCCH3)3(bpy)2] NO3 ⋅ 1.5 H2 O) and demonstrated its ORR activity in alkaline medium. The ORR activity and methanol tolerance property of the Co(III) dimer were compared with those of Ketjenblack carbon (used as support for Co(III) dimer) and commercial 20 wt% Pt/C, respectively. Since Co(III) dimer is a molecular material, its characterization by single-crystal X-ray diffraction, nuclear magnetic resonance, and infrared studies revealed the chemical structure unambiguously. Density functional theory calculation predicted the possibility of both end-on and side-on oxygen adsorption at the metal center of the Co(III) dimer.











Similar content being viewed by others
References
Kinoshita K (1992) Electrochemical oxygen technology. Wiley, New York
Yousefi S, Zohoor M (2013) Investigating the effect of operating parameters on the open circuit voltage of a passive DMFC. Ionics 19(8):1195–1201. doi:10.1007/s11581-013-0924-z
Yousefi S, Ganji DD (2012) Experimental investigation of a passive direct methanol fuel cell with 100 cm2 active areas. Electrochim Acta 85:693–699. doi:10.1016/j.electacta.2012.08.045
Shrivastava NK, Thombre SB, Mallick RK (2014) Effect of diffusion layer compression on passive DMFC performance. Electrochim Acta 149:167–175. doi:10.1016/j.electacta.2014.10.080
Yousefi S, Shakeri M, Sedighi K (2013) The effect of cell orientations and environmental conditions on the performance of a passive DMFC single cell. Ionics 19(11):1637–1647. doi:10.1007/s11581-013-0889-y
Yousefi S, Zohoor M (2014) Conceptual design and statistical overview on the design of a passive DMFC single cell. Int J Hydrog Energy 39(11):5972–5980. doi:10.1016/j.ijhydene.2014.01.117
Hashemi R, Yousefi S, Faraji M (2015) Experimental studying of the effect of active area on the performance of passive direct methanol fuel cell. Ionics 21(10):2851–2862. doi:10.1007/s11581-015-1479-y
Jasinski R (1965) Cobalt phthalocyanine as a fuel cell cathode. J Electrochem Soc 112(5):526–528. doi:10.1149/1.2423590
Othman R, Dicks AL, Zhu Z (2012) Non precious metal catalysts for the PEM fuel cell cathode. Int J Hydrog Energy 37(1):357–372. doi:10.1016/j.ijhydene.2011.08.095
Zhang L, Zhang J, Wilkinson DP, Wang H (2006) Progress in preparation of non-noble electrocatalysts for PEM fuel cell reactions. J Power Sources 156(2):171–182. doi:10.1016/j.jpowsour.2005.05.069
Yatsimirskii KB (1990) Metal complexes of macrocycles in catalysis. Russ Chem Rev 59(12):1150
Bagotzky VS, Tarasevich MR, Radyushkina KA, Levina OA, Andrusyova SI (1978) Electrocatalysis of the oxygen reduction process on metal chelates in acid electrolyte. J Power Sources 2(3):233–240. doi:10.1016/0378-7753(78)85014-9
van Veen JAR, Colijn HA (1981) Oxygen reduction on transition-metal porphyrins in acid electrolyte II. Stability Berichte der Bunsengesellschaft für Physikalische Chemie 85(9):700–704. doi:10.1002/bbpc.19810850918
van Veen JAR, van Baar JF, Kroese CJ, Coolegem JGF, De Wit N, Colijn HA (1981) Oxygen reduction on transition-metal porphyrins in acid electrolyte I. Activity. Berichte der Bunsengesellschaft für Physikalische Chemie 85(9):693–700. doi:10.1002/bbpc.19810850917
Wiesener K, Ohms D, Neumann V, Franke R (1989) N4 macrocycles as electrocatalysts for the cathodic reduction of oxygen. Mater Chem Phys 22(3–4):457–475. doi:10.1016/0254-0584(89)90010-2
Ladouceur M, Lalande G, Guay D, Dodelet JP, Dignard‐Bailey L, Trudeau ML, Schulz R (1993) Pyrolyzed cobalt phthalocyanine as electrocatalyst for oxygen reduction. J Electrochem Soc 140(7):1974–1981. doi:10.1149/1.2220748
Domínguez C, Pérez-Alonso FJ, Abdel Salam M, de la Fuente JL G, Al-Thabaiti SA, Basahel SN, Peña MA, Fierro JLG, Rojas S (2014) Effect of transition metal (M: Fe, Co or Mn) for the oxygen reduction reaction with non-precious metal catalysts in acid medium. Int J Hydrog Energy 39(10):5309–5318. doi:10.1016/j.ijhydene.2013.12.078
van Veen JAR, Colijn HA, van Baar JF (1988) On the effect of a heat treatment on the structure of carbon-supported metalloporphyrins and phthalocyanines. Electrochim Acta 33(6):801–804. doi:10.1016/S0013-4686(98)80010-8
Gupta S, Tryk D, Bae I, Aldred W, Yeager E (1989) Heat-treated polyacrylonitrile-based catalysts for oxygen electroreduction. J Appl Electrochem 19(1):19–27. doi:10.1007/BF01039385
Hung T-F, Chen S-H, Tu M-H, Lu Z-H, Chen CK, Liu R-S, Greer HF, Zhou W, Lo M-Y (2014) Advances in carbon-incorporated non-noble transition metal catalysts for oxygen reduction reaction in polymer electrolyte fuel cells. J Chin Chem Soc 61(1):93–100. doi:10.1002/jccs.201300286
Banham D, Ye S, Pei K, Ozaki J-i, Kishimoto T, Imashiro Y (2015) A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J Power Sources 285:334–348. doi:10.1016/j.jpowsour.2015.03.047
Zhu C, Li H, Fu S, Du D, Lin Y (2016) Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem Soc Rev. doi:10.1039/C5CS00670H
Artyushkova K, Serov A, Rojas-Carbonell S, Atanassov P (2015) Chemistry of multitudinous active sites for oxygen reduction reaction in transition metal–nitrogen–carbon electrocatalysts. J Phys Chem C 119(46):25917–25928. doi:10.1021/acs.jpcc.5b07653
Kramm UI, Herranz J, Larouche N, Arruda TM, Lefèvre M, Jaouen F, Bogdanoff P, Fiechter S, Abs-Wurmbach I, Mukerjee S, Dodelet J-P (2012) Structure of the catalytic sites in Fe/N/C-catalysts for O(2)-reduction in PEM fuel cells. Phys Chem Chem Phys: PCCP 14(33):11673–11688. doi:10.1039/c2cp41957b
Sahraie NR, Kramm UI, Steinberg J, Zhang Y, Thomas A, Reier T, Paraknowitsch J-P, Strasser P (2015) Quantifying the density and utilization of active sites in non-precious metal oxygen electroreduction catalysts. Nat Commun 6:8618. doi:10.1038/ncomms9618
Roelfes G, Vrajmasu V, Chen K, Ho RYN, Rohde J-U, Zondervan C, la Crois RM, Schudde EP, Lutz M, Spek AL, Hage R, Feringa BL, Münck E, Que L (2003) End-on and side-on peroxo derivatives of non-heme iron complexes with pentadentate ligands: models for putative intermediates in biological iron/dioxygen chemistry. Inorg Chem 42(8):2639–2653. doi:10.1021/ic034065p
Türk K-K, Kruusenberg I, Mondal J, Rauwel P, Kozlova J, Matisen L, Sammelselg V, Tammeveski K (2015) Oxygen electroreduction on MN4-macrocycle modified graphene/multi-walled carbon nanotube composites. J Electroanal Chem 756:69–76. doi:10.1016/j.jelechem.2015.08.014
Lattelais M, Bocquet ML (2015) Cycloaddition of metal porphines on metal-supported graphene: a computational study. J Phys Chem C 119(17):9234–9241. doi:10.1021/jp512247n
You J-M, Han HS, Lee HK, Cho S, Jeon S (2014) Enhanced electrocatalytic activity of oxygen reduction by cobalt–porphyrin functionalized with graphene oxide in an alkaline solution. Int J Hydrog Energy 39(10):4803–4811. doi:10.1016/j.ijhydene.2014.01.107
Liu Z, Anson FC (2000) Electrochemical properties of vanadium(III, IV, V)−salen complexes in acetonitrile. Four-electron reduction of O2 by V(III)−salen. Inorg Chem 39(2):274–280. doi:10.1021/ic990958z
Kryatov SV, Taktak S, Korendovych IV, Rybak-Akimova EV, Kaizer J, Torelli S, Shan X, Mandal S, MacMurdo VL, Mairata i Payeras A, Que L (2005) Dioxygen binding to complexes with FeII2(μ-OH)2 cores: steric control of activation barriers and O2-adduct formation. Inorg Chem 44(1):85–99. doi:10.1021/ic0485312
Mamuru SA, Ozoemena KI, Fukuda T, Kobayashi N, Nyokong T (2010) Studies on the heterogeneous electron transport and oxygen reduction reaction at metal (Co, Fe) octabutylsulphonylphthalocyanines supported on multi-walled carbon nanotube modified graphite electrode. Electrochim Acta 55(22):6367–6375. doi:10.1016/j.electacta.2010.06.056
Jahan M, Bao Q, Loh KP (2012) Electrocatalytically active graphene–porphyrin MOF composite for oxygen reduction reaction. J Am Chem Soc 134(15):6707–6713. doi:10.1021/ja211433h
Mo G, Liao S, Zhang Y, Zhang W, Ye J (2012) Synthesis of active iron-based electrocatalyst for the oxygen reduction reaction and its unique electrochemical response in alkaline medium. Electrochim Acta 76:430–439. doi:10.1016/j.electacta.2012.05.054
Jiang L, Li M, Lin L, Li Y, He X, Cui L (2014) Electrocatalytic activity of metalloporphyrins grown in situ on graphene sheets toward oxygen reduction reaction in an alkaline medium. RSC Adv 4(51):26653–26661. doi:10.1039/C4RA02208D
Liu K, Song Y, Chen S (2014) Electrocatalytic activities of alkyne-functionalized copper nanoparticles in oxygen reduction in alkaline media. J Power Sources 268:469–475. doi:10.1016/j.jpowsour.2014.06.054
Li M, Bo X, Zhang Y, Han C, Guo L (2014) Comparative study on the oxygen reduction reaction electrocatalytic activities of iron phthalocyanines supported on reduced graphene oxide, mesoporous carbon vesicle, and ordered mesoporous carbon. J Power Sources 264:114–122. doi:10.1016/j.jpowsour.2014.04.101
Han J, Sa YJ, Shim Y, Choi M, Park N, Joo SH, Park S (2015) Coordination chemistry of [Co(acac)2] with N-doped graphene: implications for oxygen reduction reaction reactivity of organometallic Co–O4–N species. Angew Chem Int Ed 54(43):12622–12626. doi:10.1002/anie.201504707
Fashedemi OO, Ozoemena KI (2015) Oxygen reduction reaction at MWCNT-modified nanoscale iron(ii) tetrasulfophthalocyanine: remarkable performance over platinum and tolerance toward methanol in alkaline medium. RSC Adv 5(29):22869–22878. doi:10.1039/C5RA03133H
Becke AD (1993) Density‐functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652. doi:10.1063/1.464913
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 33(12):8822–8824
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82(1):299–310. doi:10.1063/1.448975
Wang X, Wang L, Zhao F, Hu C, Zhao Y, Zhang Z, Chen S, Shi G, Qu L (2015) Monoatomic-thick graphitic carbon nitride dots on graphene sheets as an efficient catalyst in the oxygen reduction reaction. Nanoscale 7(7):3035–3042. doi:10.1039/C4NR05343E
Gasteiger HA, Kocha SS, Sompalli B, Wagner FT (2005) Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl Catal B Environ 56(1–2):9–35. doi:10.1016/j.apcatb.2004.06.021
Paulus UA, Schmidt TJ, Gasteiger HA, Behm RJ (2001) Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: a thin-film rotating ring–disk electrode study. J Electroanal Chem 495(2):134–145. doi:10.1016/S0022-0728(00)00407-1
Palanivelu KM, Prabhakaran V, Ramani VK, Ramanujam K (2015) Controlling the nitrogen content of metal–nitrogen–carbon based non-precious-metal electrocatalysts via selenium addition. J Electrochem Soc 162(6):F475–F482. doi:10.1149/2.0101506jes
Shi Z, Zhang J (2007) Density functional theory study of transitional metal macrocyclic complexes’ dioxygen-binding abilities and their catalytic activities toward oxygen reduction reaction. J Phys Chem C 111(19):7084–7090. doi:10.1021/jp0671749
Liang Y, Li Y, Wang H, Zhou J, Wang J, Regier T, Dai H (2011) Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat Mater 10(10):780–786. http://www.nature.com/nmat/journal/v10/n10/abs/nmat3087.html#supplementary-information
Wu G, Nelson M, Ma S, Meng H, Cui G, Shen PK (2011) Synthesis of nitrogen-doped onion-like carbon and its use in carbon-based CoFe binary non-precious-metal catalysts for oxygen-reduction. Carbon 49(12)):3972–3982. doi:10.1016/j.carbon.2011.05.036
Coutanceau C, Croissant MJ, Napporn T, Lamy C (2000) Electrocatalytic reduction of dioxygen at platinum particles dispersed in a polyaniline film. Electrochim Acta 46(4):579–588. doi:10.1016/S0013-4686(00)00641-1
Geniès L, Faure R, Durand R (1998) Electrochemical reduction of oxygen on platinum nanoparticles in alkaline media. Electrochim Acta 44(8–9):1317–1327. doi:10.1016/S0013-4686(98)00254-0
Gubbins KE, Walker RD (1965) The solubility and diffusivity of oxygen in electrolytic solutions. J Electrochem Soc 112(5):469–471. doi:10.1149/1.2423575
Haynes WM, Lide DR (2011) CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. CRC, Boca Raton
Bonakdarpour A, Lefevre M, Yang R, Jaouen F, Dahn T, Dodelet J-P, Dahn JR (2008) Impact of loading in RRDE experiments on Fe–N–C catalysts: two- or four-electron oxygen reduction? Electrochem Solid-State Lett 11(6):B105–B108. doi:10.1149/1.2904768
Griffith JS (1956) On the magnetic properties of some haemoglobin complexes. Proc R Soc Lond A: Math Phys Eng Sci 235(1200):23–36
Pauling L (1964) Nature of the iron–oxygen bond in oxyhaemoglobin. Nature 203(4941):182–183
Adzic R (1996) Recent advances in the kinetics of oxygen reduction. In: Lipkowski J, Ross PN (eds) Electrocatalysis. Wiley, New York, p 197–237
Xia XH, Iwasita T, Ge F, Vielstich W (1996) Structural effects and reactivity in methanol oxidation on polycrystalline and single crystal platinum. Electrochim Acta 41(5):711–718. doi:10.1016/0013-4686(95)00360-6
Kauranen PS, Skou E (1996) Mixed methanol oxidation/oxygen reduction currents on a carbon supported Pt catalyst. J Electroanal Chem 408(1996):189–198. doi:10.1016/0022-0728(96)04515-9
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 548 kb)
Rights and permissions
About this article
Cite this article
Sheelam, A., Mandal, S., Thippani, T. et al. Carbon-supported Co(III) dimer for oxygen reduction reaction in alkaline medium. Ionics 22, 2183–2194 (2016). https://doi.org/10.1007/s11581-016-1730-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1730-1