Abstract
In this paper, we present results of our research on developing of new deoxyribonucleic acid (DNA)-based conducting membranes for application in electrochromic devices. Improvements of performances of DNA-based solid bioelectrolyte in smart windows were achieved by adding plasticizer like glycerol and different amounts of photosensitive chromophores, such as Nile Blue. The results were obtained and analyzed by a variety of experimental tools and techniques such as FT-IR, UV–VIS spectroscopy, fluorescence, electric conductivity, contact angle, charge density measurements, and cyclic voltammetry. The biomembranes with the highest ionic conductivity values were successfully applied in smart windows with glass/ITO/WO3/DNA-based membranes/CeO2–TiO2/ITO/glass configuration which have shown a good change of transmittance under the applied electric field. The obtained results suggest that DNA-based electrolytes are very promising materials to be applied in electrochromic devices.
Similar content being viewed by others
Introduction
Electrochromism is a property of some materials to change their optical transmission (reflection) spectrum (coloration) under the applied external electric field. It happens due to the modification of their oxidation states [1, 2]. Usually, the materials are studied and characterized in form of electrochromic devices (ECDs), as first applications were thought in displays. Also, observation of the effect is straightforward and easily monitored. The ECDs consist of two semitransparent indium tin oxide (ITO) electrodes, providing sufficient light transmission. The space between them is filled with an electrolyte serving to transport charges from one electrode to the other [3, 4]. Some conducting polymers like polyaniline and polythiophene change also their absorption band through the change of their oxidation state via doping/dedoping processes.
Nowadays, much wider practical applications of these materials are envisaged. It concerns particular optical switching devices, electrochromic windows (ECWs), also called smart windows, controllable by electric field optical transmission [3], Graetzl-type photovoltaic cells [5], car rear-view mirrors [6], transmission-controllable sunroofs in automobiles, and transmission-controllable sunglasses. All ECDs show a reversible color change by reduction or by oxidation of the electrochromic material caused by the applied electric field [7].
The variation of the ECDs redox states are obtained by applying electric DC field between electrodes of the device. For practical application, it is important that the change of transmission is uniform over the whole surface of ECD and is reversible. Also, the lifetime of the device should be sufficiently long and its operation stable over a sufficiently, depending on application, long period of time. It should be at least few thousands of on/off switching periods [8].
At the beginning, the ECDs were made with liquid electrolytes. Such devices are not easy to fabricate and require the use of a tight assembling to contain usually highly reactive electrolyte. The emergence of solid polymer electrolyte (SPEs) [9, 10] greatly facilitated the technology, particularly for smart windows and solar cells, and made possible the fabrication of large-surface, flexible devices. It increases the power density of devices and their stability under electrochemical cycling, unlike the crystalline or vitreous materials [11, 12]. To avoid the contribution of synthetic polymers to the pollution of environment biomaterials, polysaccharides [13, 14], proteins [15], and deoxyribonucleic acid (DNA) [16] were recently proposed and successfully demonstrated their potential for application in ECDs fabrication. They are eco-friendly materials [17], originate from renewable resources, and can be cheap. If not protected, they are biodegradable. In particular, DNA, the symmetrical “molecule of life” [18], with a unique structure and the ability to act as a host capable to align itself and to induce orientation of chromophores, attracted a lot of interest for its high potentiality for application in photonics and in electronics [19–22]. DNA is a negatively charged anionic polyelectrolyte with sodium Na+ as counter ions; it exhibits an important ability to form transparent membranes with a good adhesion to steel electrodes. Its theoretical electric conductivity of DNA first suggested by Eley and Spivey [23] is high. As argued by them, it is due to the charge transfer provided by the delocalized π electrons in nucleobases and taking place along the double helix of DNA macromolecules, whereas the peripheral part of DNA molecule with sugars and phosphates behaves as an insulator. Also, due to its specific, double-strand helical structure of DNA, the charge extraction is fast, assuring a relatively short response time [3].
In this work, we describe fabrication of DNA-based membranes in view of their application in smart windows. The membranes were characterized by UV–VIS–NIR spectroscopy. Glycerine was used as plasticizer. Its influence on electric conductivity was also studied. To boost the conductivity, which is important for practical applications, Nile Blue (NB), a reputed DNA stain, was added.
The ionic conductivity of most polymer electrolytes can be improved by different approaches, such as plasticization [24, 25], cross-linking, and grafting [26, 27] processes. Those processes involve the exchange or introduction of functional groups into polymer chains, leading to a change in the gelatinization and retrogradation properties of these macromolecules. As an example, the modified starch can be obtained by introducing hydroxypropyl groups, resulting in inter- and intramolecular hydrogen bonding interruption and, consequently, an increase of the amorphous phase [28]. The polymer electrolytes obtained by the plasticization process with low molecular weight substances increase the values of the ionic conductivity of the formed blends. DNA can be functionalized with photosensitive and conduction increasing molecules, providing to the material some interesting optical and electrical properties.
The main purpose of the present research is the preparation and characterization of new DNA-based materials with high conductivity and good optical properties for fabrication of conducting membranes in view of their utilization in ECDs. The membranes were prepared by mixing aqueous solution of DNA and of glycerol (GLY), used as plasticizer. They were doped with a photosensitive NB [29] (Fig. 1) chromophore at different levels in order to increase the membranes’ ionic conductivity and to improve their optical properties. Also, in order to study how the presence of some simple intercalators or dopants into membranes composition influences the conductivity and optical absorption properties of these DNA-based membranes, the ionic doping molecules such as NB were added in different concentrations. The heterocyclic planar and rigid structure of this chromophore is expected promote its intercalation NB into the relatively non-planar structure of DNA helix macromolecule [30]. In fact, some literature suggests bonding of NB to DNA by both electrostatic and intercalative interactions [29]. It is expected that the interaction of NB with DNA can influence the ionic conductivity of the membranes.
Usually, GLY is widely used as plasticizers of biopolymers. The mechanism is not well known yet. It is assumed that plasticizer separates the polymer chain increasing in this way the ionic conductivity [31]. The interactions between natural polymer and plasticizer can be explained by either the Van der Waals forces and/or by formation of hydrogen bonds due to polymer–polymer and polymer–plasticizer interactions [24].
The paper is organized as follows: in Chapter 2, we list the materials used and describe the preparation technology and the experimental techniques used in membranes characterization; the obtained results are presented and discussed in Chapter 3 and the last chapter (4) gives conclusion of the study.
Materials and experimental methods
Materials and membranes fabrication
In this study, we used DNA extracted from waste produced by salmon food processing industry. It was purchased in Japan, from Ogata Research Laboratory, Ltd., Chitose. The average molecular weight (MW) of the native DNA used was 2 × 107 g mol−1. The NB chromophore used was purchased from Sigma-Aldrich. For electrochromic devices as electrodes, WO3/ITO/glass and CeO2–TiO2/ITO/glass were used, as described in literature [3, 32].
The DNA-based membranes were prepared at room temperature by casting technique from a homogenous mixture containing aqueous solution of DNA and of glycerol, used as plasticizer, doped with chromophore, such as NB. The technique for obtaining thin biomembranes consists of the following steps: first, the water solution of glycerol is mixed with chromophore (NB) for more than 12 h. Then glycerol and chromophore mixture is poured in water solution of DNA and stirred for another 12 h to ensure formation of a homogenous gel. To obtain a total dissolution of chromophore, the stirring is accompanied by an occasional ultrasonic process. Finally, the obtained gel is poured in Teflon boxes and placed in an oven at 50 °C for more than 3 days. The resultant membranes have a thickness ranging from 0.04 to 0.5 mm.
The mass ratio DNA/GLY was varied from 10:1 to 6:1 and 1:1. The chromophore concentration was maintained constant at 0.5 wt%, relative to that of DNA/GLY (Table 1). The ionic conductivity measurements have shown that the DNA/GLY 1:1 biomembranes exhibit the highest conductivity. In order to find a proper chromophore concentration to get the best conductivity, in the next step, the ratio DNA/GLY was kept constant at 1:1, and the chromophore concentration was varied from 0.5 to 1, 2, and 3 wt%, as reported in Table 1.
Membranes characterization
The Fourier transform infrared (FT-IR) measurements were performed at room temperature with a Perkin Elmer Spectrum 100 FT-IR spectrometer in the attenuated total reflection mode. All spectra were recorded between 4,000 and 600 cm−1 with a resolution of 4 cm−1 and four acquisitions per sample.
UV–VIS spectra for membranes and also for ECDs based on membranes with the highest ionic conductivity value were recorded using a Jasco UV–VIS spectrophotometer, model V 670. Fluorescence spectra of the new DNA-based membranes were recorded on a JASCO FP 6500 spectrofluorometer with an excitation wavelength of 616 nm.
In order to check influence of wettability on ionic conductivity of DNA-based membranes, contact angle measurements were performed using a contact angle meter—KSV Instruments apparatus, model CAM 100. The investigation was carried out with the accuracy of ±1° at 25 °C.
The ionic conductivity measurements for the prepared ECDS were carried out by electrochemical impedance spectroscopy using the Autolab PGSTAT 302N potentiostat with general-purpose electrochemical system software. The biopolymeric electrolyte with 2 cm diameter and 0.04–0.5 mm thickness was pressed between two stainless steel electrodes with surface of 1 cm2. The measurements were conducted at free potential, in the frequency range of 106 Hz to 10 mHz and with the applied voltage amplitude of 10 mV.
The ECDs containing the DNA-based membranes were also characterized by cyclic voltammetry studies performed with the Autolab PGSTAT 302N device, applying a tension ranging from −2 to 2 V with the electric field scan step of 100 mV s−1.
Electrochromic smart windows (ESWs) fabrication
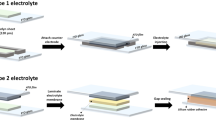
The electrochromic devices used for smart windows application, with the glass/ITO/WO3/DNA-based electrolyte/CeO2–TiO2/ITO/glass, were built as described in literature [3, 33] by depositing a DNA-based membrane on the ITO-coated glass substrate with ITO/WO3 films and almost 1 cm free space was left for electrical contact from Cu-conducting tape. Then, the CeO2–TiO2/ITO/glass substrate was pressed onto the membrane in such a way that the two coatings faced each other inside the assembled window.
Results and discussion
FT-IR spectroscopy
To examine the interaction of DNA with NB chromophore, the infrared spectra of all raw materials used (Fig. 2) and of all obtained biomembranes based on DNA, GLY, and NB in different concentrations (Fig. 3) were recorded.
Those recorded for raw DNA and DNA membrane are similar (Figs. 2 and 3). In the four DNA bases, adenine, cytosine, guanine, and thymine, a variety of chemical bonds like C=O, N–H, C–H, O–H, and C–O are present. They absorb the infrared light through bending, scissoring, or stretching vibrations. The absorption band corresponding to the stretching vibrations of N–H (νN-H) and O–H (νO-H) bonds appears at around 3,168 cm−1. At 1,682 and 1,647 cm−1, the bands attributed to the C=O (νC=O from Amide I) stretching vibration are observed. A deformation of N–H vibration is present in the region of 1,576–1,527 cm−1 (δNH from Amide II). In the frequency range 1,478–1,416 cm−1, bands assigned to the deformation vibration of CH2 appear, and between 1,365 and 1,334 cm−1 the bands attributed to deformation vibration of CH3 are noticed. Also, two strong bands are present. The first one at 1,230 cm−1 is attributed to asymmetric PO2 − stretching vibration (νPO2 − asm) and the second at 1,060 cm−1 corresponds to symmetric PO2 − stretching vibration (νPO2 − sm) [34–36] in DNA.
The IR spectrum recorded for the NB powder presents the following main bands: a band at 3,110 cm−1 is attributed to the =C–H stretches of aromatics. Bands at 2,935 and at 2,873 cm−1 correspond to νC-H and νCH3. The band at 1,583 cm−1 is assigned to the stretching vibration of C=C (νC=C) bond and the absorption band observed at 1,547 cm−1 corresponds to the aromatic substitution. The absorption bands between 1,500 and 1,423 cm−1 are due to the carbon–carbon stretches in the aromatic ring. The band at 1,482 cm−1 corresponds to the deformation vibration of CH2. The bands in 1,374–1,332 cm−1 range are attributed to the deformation vibration of CH3. The band at 1,191 cm−1 is attributed to C–O bond from aromatic cycle and at 1,067 cm−1 the in-plane C–H bending is seen.
Figure 2 shows also the infrared absorption spectrum of glycerol. The absorption peak at 3,292 cm−1 is attributed to O–H stretching (νO-H) [37]. The C–H stretching (νC-H) band is seen at 2,932 and 2,879 cm−1, while the C–O–H bending is observed at 1,452 and 1,416 cm−1. The bands in the region of 1,452–1,031 cm−1 correspond to C–O bond from aromatic cycle. The O–H bending is seen at 922 cm−1.
The infrared spectra of the DNA-based membranes with or without GLY and chromophore are shown in Fig. 3. They reveal the characteristic absorption bands of each component and the hydrogen bonds formed between molecules.
In all IR spectra registered for biomembranes (Fig. 3), the band corresponding to the stretching vibration of associated N–H (νN-H) from DNA and to O–H (νO-H) from DNA and GLY appear at the 3,281–3,180 cm−1 frequency range. This band is also attributed to the intermolecular hydrogen bonds formation between hydroxyl groups from GLY and phosphate groups from double-stranded DNA. The bands at 1,687 and 1,642 cm−1 are attributed to the stretching vibration (νC=O Amide I) of C=O and to NH2 scissoring vibrations from DNA membrane. These bands are observed in all membranes at other wavenumbers ranges: 1,706–1,682 cm−1 and 1,654–1,638 cm−1. The band at 1,527 cm−1, corresponding to the deformation vibration of N–H bond from DNA membrane (δNH Amide II), is present in all studied biomembranes at 1,530 cm−1 with almost the same intensity.
The band corresponding to the asymmetric PO2 − stretching vibration (νPO2 − asm) in DNA is present at the same wavenumber (1213 cm−1) in all biomembranes with DNA, GLY, and NB at different concentrations. The band assigned to symmetric PO2 − stretching vibration (νPO2 − sm) from DNA membrane is found in all membranes between 1040 – 1037 cm−1. The interaction between plasticizer and DNA leads to separation of large double-stranded DNA according to the literature, creating free spaces between the polymer chains [38].
The absorption band at 1,583 cm−1 in IR spectrum of NB, assigned to stretching vibration of C=C (νC=C) from aromatic ring, is also present in all NB containing biomembranes in wavenumber range of 1,584–1,579 cm−1. Existence of this band proves that the new vacant gaps created by the presence of plasticizer lead to intercalation of NB between the two separated DNA chains [29].
UV–VIS spectroscopy
The optical absorption spectra of fabricated biomembranes were collected in the wavelength range 200–800 nm. The measurements were done with the scanning step of 0.5 nm. Figure 4 shows some examples of the measured absorption spectra of some DNA-based membranes with different mass ratios between DNA and GLY and for the same concentration of NB.
In all cases, the absorption peak at around 650 nm, characteristic of the NB molecule, is observed.
Fluorescence spectroscopy
NB is known to be an efficient luminophore. To see whether the photoluminescence is present also in membranes, we have measured their fluorescence spectra with an excitation wavelength of 616 nm. Figure 5 displays the fluorescence spectra for DNA-based membranes with different concentrations of NB. They show the maximum emission of NB chromophore around 670 nm, with a maximum intensity for biomembrane with 0.5 wt% NB. At higher concentration (3 wt%), a smaller fluorescence intensity is observed that is due to its quenching by the luminophore aggregation.
Electric conductivity
The required main property of DNA-based membrane in view of their application in ECDs is a good ionic conductivity. It was measured at room temperature for different DNA-based membranes plasticized or not with GLY, and doped or not with NB chromophore. They are displayed in Fig. 6.
Adding glycerol as plasticizer to the DNA membrane composition promotes an improvement of ionic conductivity from 5.65 × 10−9 to 9.02 × 10−9 S cm−1. This result is in good agreement with that reported by Pawlicka et al. [39], which shows that the presence of GLY in the membrane composition leads to obtaining transparent membranes with improved ionic conductivities. In case of the membranes which contain only DNA and 2 wt% of NB, the ionic conductivity of membrane decreases. This is due to the fact that the ionic mobility is smaller and the plasticization process is necessary. The improvement of the ionic conductivity of DNA-based membranes by adding NB chromophore besides GLY can be explained by the fact that NB is a good intercalator of the DNA double helix and causes important structural transformation from DNA [40]. Ju et al. argue that between DNA solution and NB molecules, some electrostatic and intercalative interactions occur, which has an important influence on ionic conductivity behavior [29]. In our case, these interactions are highly improved due the presence of plasticizer, which separates the double helix of DNA by breaking the hydrogen bonding in nitrogenous bases [38] and creating vacant gaps for NB intercalation.
The quantity of chromophore added to DNA plays an important role in ionic conductivity obtained. In order to evaluate the influence of the chromophore concentration on ionic conductivity, we measured as function of temperature in the temperature range of 20–100° for DNA-based electrolytes samples plasticized with GLY. Samples with the following NB concentrations were prepared and studied: 0.5, 1, 2, and 3 wt%, and the obtained results are reported in Table 2.
All DNA-based membranes with NB exhibit ionic conductivities ranging from 10−9 to 10−8 S cm−1 at room temperature. The ionic conductivity of membranes increases with temperature and reaches a value of about 10−5 S cm−1 at the temperature close to 80 °C. This behavior of membranes conductivity at temperatures higher than 70 °C is probably due to the denaturation of DNA, which produces free guanine and adenine. It is provoked by the rupture of hydrogen bonds between the double-stranded DNA macromolecules, which are partly separated into two single-stranded DNA [41]. Thus, as a consequence, the number of mobile charge carriers and the delocalization of π electrons in nucleobases increase.
Concerning the influence of NB content on ionic conductivity of DNA-based membranes, the highest values were observed in the case of membranes with 2 wt% of NB. Addition of more NB, i.e., 3 wt%, decreases it down to 4.35 × 10−9 S cm−1 at room temperature. This is essentially due to the formation of less mobile chromophore aggregates. As a result, the number of mobile charge carriers decreased. In some cases, we observed a decrease in ionic conductivity at temperatures higher than 80 °C. This is due to the evaporation of glycerol and, thus, the reduced number of charge carriers.
Contact angle measurement
The wettability of the final obtained membranes can give information about the hydrophilic/hydrophobic interaction between NB, GLY, and DNA. Thus, the wettability of DNA-based membranes with different compositions was evaluated by measuring the contact angle formed between the water drop and the biomembrane surface. The volume of the liquid used was kept constant (10 μL) in all contact angle measurements, and the image of liquid drop was recorded by video camera. Each contact angle value is the average of minimum of five measurements. The wettability of the studied biomembranes is in the range of θ = 93–146° (Fig. 7). The observed behavior of contact angle in studied membranes can be explained by interaction between the molecules (NB, GLY) with hydrophilic phosphate groups and both hydrophobic and hydrophilic amine bases groups from DNA [42]. The GLY addition into the DNA solution promotes hydrophilic interaction around the phosphate groups, thereby stabilizing the helix hydrophobic phase. The contact angle measurements show a hydrophobic character of DNA-based membranes, which increases when GLY is added and decreases with chromophore addition into the biomembranes composition. The hydrophobic part of NB molecules intercalate with hydrophobic phase from DNA, stabilizing the hydrophilic phosphate phase, thus improving the hydrophilic character of the membrane. When adding different percentage of NB into the composition, a slight difference in contact angle values is observed when the NB concentration is increased to 2 and 3 wt%, respectively.
The hydrophilic/hydrophobic character depends of the interaction between molecules which plays an important role in obtaining of membranes with good conductivities. Comparing the wettability results with the ionic conductivity measurements, one can conclude that increasing the chromophore concentration in DNA + GLY biomembranes enhances their wettability behavior, leading at the same time to increase of their conductivity.
Charge density measurements
In order to verify the performance of the biomembranes in electrochemical devices, small electrochromic windows with glass/ITO/WO3/DNA-based membranes/CeO2–TiO2/ITO/glass configuration were assembled, and the charge density values were measured by chronoamperometry by applying different negative potentials values and different times. The obtained results are displayed in Fig. 8a and b. In Fig. 8a, one can see that the inserted charge increases with the applied negative potential. It reaches −0.5 mC · cm−2 in 10 s at −1.5 V applied voltage and increased to −1.3 mC · cm−2 in 10 s at −2.8 V, respectively. In all the studied cases, the charge extraction is faster than insertion and the ECD changes color already after applying +2.0 V potential during 2 s. Moreover, the inserted charge increases (Fig. 8b) with time at the applied potential of −2.8 V/2 V from −2.1 mC · cm−2 at 15 s/15 s to −3 mC · cm−2 at 30 s/30 s and to −4.1 mC · cm−2 at 60 s/60 s, respectively.
After applying a negative potential of −2.8 V on the device made with DNA1GLY1NB2 membrane, a change of color intensity (Fig. 9) was observed according to the reaction WO3 (bleached) + xe− + xM+ ↔ MxWO3 (intense blue color). The tungsten trioxide electrode is blue when it is polarized cathodically by simultaneous electrons and ions insertion, and it is colorless when it is polarized anodically (+2 V), associated with charge extraction.
In order to check the reversibility of the ECD, the samples were subjected to chronoamperometric cycling by applying potentials of −2.8 V and +2 V for 15 s/15 s. In Fig. 10, the charge densities measured after 1, 100, and 600 cycles are presented.
During the test, a change of color occurred between intense blue and colorless, and the charge density value decreased with the number of cycles. After 100 cycles, the charge density decreased from −2.16 to −2.06 mC · cm−2. After 600 cycles, these values decreased to −1.47 mC · cm−2. This behavior is in accordance with results obtained from transmittance spectra (Fig. 12), indicating a decrease in time of the DNA1GLY1NB2 membrane transmittance change.
Cyclic voltammetry (CV)
Typical cyclic voltammograms of studied ECD containing DNA1GLY1NB2 membrane at different scan rates are shown in Fig. 11. In all cases of CVs at different scan rates (Fig. 11a), one cathodic peak with a maximum centered at −1.64 V (for 500 mV · s−1) is observed, which is associated with the blue color process due to the reduction of WO3 coating, and it is explained as an intervalence optical charge-transfer transition between adjacent WVI and WV canters. Also, the reversible reaction of bleaching is confirmed by a well-defined anodic peak, centered at −0.18 V (for 500 mV · s−1). The second anodic peak at 0.546 V, which was directly proportional to the scan rate, is associated to electrolyte oxidation, in which, according to literature [29], a quasi-reversible process occurs, with a two-electron and two-proton transfer, indicating that NB is bounded to DNA chain by electrostatic and intercalative interactions.
The studied ECD system is an irreversible one due to its dependence on the scan rate. This behavior is confirmed by other results [43]. Increasing the scan rate (Fig. 11a) of the studied system promotes an increase of the oxidation peak current and its shift to lower potentials. Plots of the catholic and anodic peak currents against the square root of scan rate are shown in Fig. 11b. They show that the peak current is proportional to the square root of the scan rate. It indicates that the mass transfer to the electrode surface is controlled by diffusion [43].
ECD transmittance
The UV–VIS transmission spectra of ECD with DNA1GLY1NB2 electrolyte and in the 190–1,100 nm range are shown in Fig. 12 after 1, 100, and 600 cycles of charge insertion/extraction cycling between −2.8 V/+2 V for 15 s/15 s. At −2.8 V applied potential, the transmittance is of 16 % at 780 nm. At this voltage, the device exhibits an intense blue color (left-hand side of Fig. 12). The increase in applied voltage to positive values moves WO3 to its oxidized state, and the transmittance increases to 21 %. The ECD exhibits a brighter blue color than in the preceding case (right-hand side of Fig. 12).
The band present in UV–VIS spectra at 550 nm corresponds to the absorption by WO3. The aforementioned transmittance difference, measured at 780 nm, is due to the presence of NB in membrane, thus originates from the solid electrolyte.
The UV–VIS spectra are in agreement with the results obtained from CV and chronoamperometry measurements concerning the reversibility of studied ECDs. It was observed that after 600 cycles of insertion/extraction charge, the color intensity decreases, leading to 5 % transmittance change. This is most likely due to the unprotected membrane oxidation.
Conclusions
In this present paper, we have presented results of our work concerning the fabrication and characterization of DNA-based conducting membranes for application in smart windows. For that, the membranes should exhibit several properties, such as facility in fabrication, and good ionic conductivity and charge extraction. As DNA plasticizer, we used glycerine. The membranes feasibility and their quality depend strongly on the substrate used and its wettability by the DNA–GLY complex. Depending on complex substrates, hydrophobic or hydrophilic substrates should be used. To increase the membranes conductivity, we used NB chromophore. Addition of this chromophore was expected to result in modification of the DNA–GLY and DNA–GLY complex–substrate interaction. To check it, a series of contact angle measurements were performed on samples containing different NB concentrations. The obtained results were correlated with FT-IR and membranes conductivity measurements. These measurements allowed to establish not only the hydrophilic/hydrophobic character of membranes but also give information about the type of interaction between DNA and GLY and between DNA–GLY complex as well as the doping NB molecule. In particular, we have found that the double-stranded DNA separation occurs due to the interaction of GLY with DNA phosphate groups. It leads to the breaking of hydrogen bonding in nitrogenous bases and reorientation of DNA chains in micelles with hydrophobic groups pushed outside, resulting in a larger contact angle. We observed also that the GLY addition favors the formation of vacant gaps and the intercalation of NB dopant, which binds electrostatically to nitrogenous bases. In this case, the DNA chains are moving in micellar structures with hydrophilic groups pushed outside. As a result, the contact angle is smaller.
From the conductivity measurements, correlated with contact angle measurements, we conclude that using GLY in DNA-based membranes composition determines a hydrophobic character and provides a higher conductivity in comparison with DNA membrane without GLY. Addition of NB to membranes made of DNA–GLY complex decreases their hydrophobic character, resulting in an increase of their conductivity. This is due to the increase of the mobile charge carriers. We have observed also that the membrane conductivity depends on the concentration of NB dopant. This is because less mobile chromophore molecules form aggregates.
Using the conducting membranes, we fabricated smart windows which exhibit a noticeable transmission variation under the applied voltage changing between 21 % and 16 % as seen in Fig. 12.
The presence of the glycerol as plasticizer and the amount of NB added in DNA membranes influence the ionic conductivity, the best value from this point of view being obtained in the case of DNA1GLY1NB2 membrane, which has also the higher wettability.
The 600 chronoamperometric cycles performed between −2.8 and +2 V have shown a decrease of the charge density, from −2.16 to −1.47 mC · cm−2.
The results presented and described in this paper show that the DNA-based electrolytes are very promising materials to be applied in electrochromic devices.
References
Mortimer RJ, Dyer AL, Reynolds JR (2006) Electrochromic organic and polymeric materials for display applications. Displays 27:2–18
Granqvist CG (1995) Handbook of inorganic electrochromic materials. Elsevier, Amsterdam
Firmino A, Grote JG, Kajzar F, Rau I, Pawlicka A (2011) Application of DNA in electrochromic cells with switchable transmission. Nonlinear Opt Quant Opt 43:181–201
Heckner KH, Kraft A (2002) Similarities between electrochromic windows and thin film batteries. Solid State Ionics 152–153:899–905
Grätzel M (2003) Dye-sensitized solar cells. J Photochem Photobioll C 4:145–153
Rosseinsky D, Mortimer RJ (2001) Electrochromic systems and the prospects for devices. Adv Mater 13:783–793
Somani PR, Radhakrishnan S (2003) Electrochromic materials and devices: present and future. Mater Chem Phys 77:117–133
Huang LM, Hu CW, Liu HC, Hsu CY, Chen CH, Ho KC (2012) Photovoltaic electrochromic device for solar cell module and self-powered smart glass applications. Sol Energ Mat Sol C 99:154–159
Şenel M, Tülü M, Bozkurt A (2007) Synthesis and proton conductivity of anhydrous dendritic electrolytes. Cent Eur J Chem 5:546–556
Schab-Balcerzak E, Grabiec E, Jarząbek B, Grobelny Ł, Kurcok M, Mazurak Z (2006) Novel soluble aromatic poly(amideimide)s containing 9,9-diphenylfluorene moieties: characterization and optical properties. Cent Eur J Chem 4:604–619
Neyertz S, Brown D (1996) Local structure and mobility of ions in polymer electrolytes: a molecular dynamics simulation study of the amorphous PEOxNaI system. J Chem Phys 104:3797–3809
Barbosa PCS, Smith MM, Gonçalves MJA, Fortunato E (2008) Solid-state electrochromic devices based on poly(trimethylene carbonate) and lithium salts. Thin Solid Films 516:1480–1483
Marcondes RFMS, D’Agostinia PS, Ferreira J, Girotto EM, Pawlicka A, Dragunski DC (2010) Amylopectin-rich starch plasticized with glycerol for polymer electrolyte application. Solid State Ionics 181:586–591
Fuentes S, Retuert PJ, Gonzalez G (2007) Lithium ion conductivity of molecularly compatibilized chitosan–poly(aminopropyltriethoxysilane)-poly(ethylene oxide) nanocomposites. Electrochim Acta 53:1417–1421
Vieira DF, Pawlicka A (2010) Optimization of performances of gelatin/LiBF4-based polymer electrolytes by plasticizing effects. Electrochim Acta 55:1489–1494
Ohno H, Takizawa N (2000) Ion conductive and flexible DNA films. Chem Lett 29:642–643
Florjanczyk Z, Debowski M, Chwojnowska E, Lokaj K, Ostrowska J, Florjanczyk Z (2009) Synthetic and natural polymers in modern polymeric materials. Part I. Polymers from renewable resources and polymer nanocomposites. Polimery 54:691–705
Steckl AJ, Spaeth H, You H, Gomez E, Grote J (2011) DNA as an optical material. Opt Photonics News 22:34–39
Singh TB, Sariciftci NS, Grote JG (2010) Bio-organic optoelectronic devices using DNA. Adv Polymer Sci 223:189–212
Bazan GC, Zalar P, Kamkar D, Naik R, Ouchen F, Grote JG, Nguyen TQ (2011) Regioregular pyridal[2,1,3]thiadiazole π-conjugated copolymers. J Am Chem Soc 133:18538–18541
Crick FHC, Watson JD (1954) The complementary structure of deoxyribonucleic acid. Proc Roy Soc London Ser A 223:80–96
Rau I, Tane A, Zgarian R, Meghea A, Grote JG, Kajzar F (2012) Stability of selected chromophores in biopolymer matrix. Mol Cryst Liq Cryst 554:43–55
Eley DD, Spivey DI (1962) Semiconductivity of organic substances. Part 9—nucleic acid in the dry state. Trans Faraday Soc 58:411–415
Galliard T (1987) Starch: properties and potentials. In: John Wiley & Sons (ed), New York
Van Soest JJG, Vliegenthart FGJ (1997) Crystallinity in starch plastics: consequences for material properties. Trends Biotechnol 15:208–213
Pawlicka A, Firmino A, Vieira D, Grote JG, Kajzar F (2009) Gelatin- and DNA-based ionic conducting membranes for electrochromic devices. Proc. of SPIE 7487 74870 J-1–74870 J-10
Dragunski DC, Pawlicka A (2001) Preparation and characterization of starch grafted with toluene poly(propylene oxide) diizocyanate. Mater Res 4:1–6
Singh J, Kaur L, McCarthy OJ (2007) Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—a review. Food Hydrocoll 21:1–22
Ju H, Ye Y, Zhu Y (2005) Interaction between Nile Blue and immobilized single- or double-stranded DNA and its application in electrochemical recognition. Electrochim Acta 50:1361–1367
Chen QY, Li DH, Zhao Y, Yang HH, Zhu QZ, Xu JG (1999) Interaction of a novel red-region fluorescent probe, Nile Blue, with DNA and its application to nucleic acids assay. Analyst 124:901–906
Chung SH, Heitjans P, Winter R, Bzauchac W, Florjanczyk Z, Onodab Y (1998) Enhancement of ionic conductivity by the addition of plasticizers in cationic monoconducting polymer electrolytes. Solid State Ionics 112:153–159
Avellaneda CO, Pawlicka A (1998) Preparation of transparent CeO2–TiO2 coatings for electrochromic devices. Thin Solid Films 335:245–248
Lima E, Raphael E, Sentanin F, Rodrigues LC, Ferreira RAS, Carlos LD, Silva MM, Pawlicka A (2012) Photoluminescent polymer electrolyte based on agar and containing europium picrate for electrochemical devices. Mater Sci Eng B 177:488–493
Pohle W, Selle C, Gauger DR, Zantl R, Artzner F, Radler JO (2000) FTIR spectroscopic characterization of a cationic lipid–DNA complex and its components. Phys Chem Chem Phys 2:4642–4650
Lindqvist M, Sarkar M, Winqvist A, Rozners E, Stromberg R, Graslund A (2000) Optical spectroscopic study of the effects of a single deoxyribose substitution in a ribose backbone: implications in RNA–RNA interaction. Biochemistry 39:1693–1701
Le-Tien C, Lafortune R, Shareck F, Lacroix M (2007) DNA analysis of a radiotolerant bacterium Pantoea agglomerans by FT-IR spectroscopy. Talanta 71:1969–1975
Cardenas G, Miranda SP (2004) FTIR and TGA studies of chitosan composite films. J Chil Chem Soc 49:291–295
Liang D, Song L, Chen Z, Chu BJ (2001) Effect of glycerol-induced DNA conformational change on the separation of DNA fragments by capillary electrophoresis. Chromatogr A 931(1–2):163–173
Pawlicka A, Donoso JP (2010) Polymer electrolytes based on natural polymers. In: Sequeira CAC, Santos DMF (eds) Polymer electrolytes: properties and applications. Woodhead, Cambridge, pp 95–128
Lakowicz JR, Piszczek G, Kang JS (2001) On the possibility of long-wavelength long-lifetime high-quantum-yield luminophores. Anal Biochem 288:62–75
Apilux A, Tabata M, Chailapakul O (2007) Electrochemical behaviors of native and thermally denatured fish DNA in the presence of cytosine derivatives and porphyrin by cyclic voltammetry using boron-doped diamond electrode. Bioelectrochemistry 70:435–439
Hammouda B, Worcester D (2006) The denaturation transition of DNA in mixed solvents. Biophys J 91(6):2237–2242
Neto MJ, Leones R, Sentanin F, Esperança JMSS, Medeiros MJ, Pawlicka A, Silva MM (2014) Ionic liquids for solid-state electrolytes and electrosynthesis. J Electroanal Chem 714–715:63–69
Acknowledgments
The authors acknowledge the financial support of Romanian Ministry of Education, Research, Youth and Sports, through the UEFISCDI organism, under Contract Number 279/7.10.2011, Code Project PN-II-ID-PCE-2011-3-05053. They would like to acknowledge also the EU support for this work within the FP7 program, grant number FP7 PIRSES-GA-2009-247544, project BIOMOLEC: Functionalized biopolymers for application in molecular electronics and in photonics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mîndroiu, M., Zgârian, R.G., Kajzar, F. et al. DNA-based membranes for potential applications. Ionics 21, 1381–1390 (2015). https://doi.org/10.1007/s11581-014-1293-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1293-y