Abstract
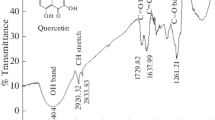
The adsorption and corrosion inhibition behavior of strawberry fruit extract at steel/1 M HCl and steel/0.5 M H2SO4 interfaces were investigated using gravimetric and electrochemical methods at 25 and 60 °C. Results obtained showed that the extract inhibited the acid-induced corrosion of mild steel which was more pronounced in HCl than H2SO4 medium. Inhibition efficiency increased with increase in the concentration of the extract but decreased with increase in temperature. The potentiodynamic polarization studies revealed that the extract functions as mixed-type inhibitors. The inhibition was assumed to occur via adsorption of the components of the extract on the mild steel surface which can be approximated by Langmuir adsorption isotherm model. Quantum-chemical calculations and molecular dynamics simulations have been used to provide insights into the mechanism of interaction of ascorbic acid as a major constituent of the extract with mild steel.











Similar content being viewed by others
References
Raja PB, Qureshi AK, Abdul Rahim A, Osman H, Awang K (2013) Neolamarckia cadamba alkaloids as eco-friendly corrosion inhibitors for mild steel in 1 M HCl media. Corros Sci 69:292–301
Oguzie EE, Oguzie KL, Akalezi CO, Udeze IO, Ogbulie JN, Njoku VO (2013) Natural products for materials protection: corrosion and microbial growth inhibition using Capsicum frutescens biomass extracts. ACS Sustain Chem Eng 1:214–225
Okafor PC, Ikpi ME, Uwah IE, Ebenso EE, Ekpe UJ, Umoren SA (2008) Inhibitory action of Phyllanthus amarus extract on the corrosion of mild steel in acid media. Corros Sci 50:2310–2317
Okafor PC, Ebenso EE, Ekpe UJ (2010) Azadirachta indica extracts as corrosion inhibitor for mild steel in acid medium. Int J Electrochem Sci 5:973–998
Oguzie EE (2008) Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corros Sci 50:2993–2998
Oguzie EE, Enenebeaku CK, Akalezi CO, Okoro SC, Ayuk AA, Ejike EN (2010) Adsorption and corrosion-inhibiting effect of Dacryodis edulis extract on low-carbon-steel corrosion in acidic media. J Coll Interf Sci 349:283–292
Deng S, Li X (2012) Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros Sci 55:407–415
Li X, Deng S, Fu H (2012) Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corros Sci 62:163–175
Chidiebere MA, Ogukwe CE, Oguzie KL, Eneh CN, Oguzie EE (2012) Corrosion inhibition and adsorption behavior of Punica granatum extract on mild steel in acidic environments: experimental and theoretical studies. Ind Eng Chem Res 51:668–677
Oguzie EE, Iheabunike ZO, Oguzie KL, Ogukwe CE, Chidiebere MA, Enenebeaku CK, Akalezi CO (2013) Corrosion inhibiting effect of Aframomum melegueta extracts and adsorption characteristics of the active constituents on mild steel in acidic media. J Dispersion Sci Technol 34:516–527
Krishnaveni K, Ravichandran J, Selvaraj A (2014) Inhibition of mild steel corrosion by Morinda tinctoria leaves extract in sulphuric acid medium. Ionics 20:115–126
Umoren SA, Gasem ZM, Obot IB (2013) Natural products for materials protection: inhibition of mild steel corrosion by date palm seed extracts in acid media. Ind Eng Chem Res 52:14855–14865
Abdel-Gaber AM, Abd-El-Nabey BA, Sidahmed IM, El-Zayady AM, Saadawy M (2006) Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros Sci 48:2765–2779
Behpour M, Ghoreishi SM, Khayatkashani M, Soltani N (2011) The effect of two oleo-gum resin exudate from Ferula assa-foetida and Dorema ammoniacum on mild steel corrosion in acidic media. Corros Sci 53:2489–2501
Quraishi MA, Singh A, Singh VK, Yadav DK, Singh AK (2010) Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater Chem Phys 122:114–122
Behpour M, Ghoreishi SM, Khayatkashani M, Soltani N (2012) Green approach to corrosion inhibition of mild steel in two acidic solutions by the extract of Punica granatum peel and main constituents, Mater. Chem Phys 2012(122):621–633
Ji G, Dwivedi P, Sundaram S, Prakash R (2013) Inhibitive effect of Chlorophytum borivilianum root extract on mild steel corrosion in HCl and H2SO4 solutions. Ind Eng Chem Res 52:10673–10681
Garai S, Garai S, Jaisankar P, Singh JK, Elango A (2012) A comprehensive study on crude methanolic extract of Artemisia (Asteraceae) and its active component as effective corrosion inhibitors of mild steel in acid solution. Corros Sci 60:193–204
Mayakrishnan G, Pitchai S, Raman K, Vincent AR, Nagarajan S (2011) Inhibitive action of Clematis gouriana extract on the corrosion of mild steel in acidic medium. Ionics 17:843–852
Kamal C, Sethuraman MG (2012) Caulerpin-A bis-indole alkaloid as a green inhibitor for the corrosion of mild steel in 1 M HCl solution from the marine alga Caulerpa racemosa. Ind Eng Chem Res 51:10399–10407
Raja PB, Fadaeinasab M, Qureshi AK, AbduRahim A, Osman H, Litaudon M, Awang K (2013) Evaluation of green corrosion inhibition by alkaloid extracts of Ochrosia oppositifolia and Isoreserpiline against mild steel in 1 M HCl medium. Ind Eng Chem Res 52:10582–10593
Ostovari A, Hoseinieh SM, Peikari M, Shadizadeh SR, Hashemi SJ (2009) Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (lawsone, gallic acid, α-d-glucose and tannic acid). Corros Sci 51:1935–1949
Giampieri F, Tulipani SJ, Alvarez-Suarez M, Quiles JL, Mezzetti B, Battino M (2012) The strawberry: composition, nutritional quality, and impact on human health—a review. Nutrition 28:9–19
Aaby K, Skrede G, Wrolstad RE (2005) Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J Agric Food Chem 53:4032–4040
Valek L, Martinez S, Mikulić D, Brnardić I (2008) The inhibition activity of ascorbic acid towards corrosion of steel in alkaline media containing chloride ions. Corros Sci 50:2705–2709
Conclaves RS, Mello LD (2001) Electrochemical investigation of ascorbic acid adsorption on low-carbon steel in 0.50 M Na2SO4 solutions. Corros Sci 43:457–470
Ferreira ES, Giacomelli C, Giacomelli FC, Spinelli A (2004) Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater Chem Phys 83:129–134
Sekine I, Nakahata Y, Tanabe H (1988) The corrosion inhibition of mild steel by ascorbic and folic acids. Corros Sci 28:987–1001, SAO SEKINE, YOSHIROU NAKAHATA and HIROSH1 TANABE
Hegazy MA, Badawi AM, Abd El Rehim SS, Kamel WM (2013) Corrosion inhibition of carbon steel using novel N-(2-(2-mercaptoacetoxy)ethyl)-N,N-dimethyl dodecan-1-aminium bromide during acid pickling. Corros Sci 69:110–122
(2011) Materials Studio. Revision 6.0. San Diego, USA, Accelrys Inc
Frenkel D, Smit B (2002) Understanding molecular simulation: from algorithms to applications, 2nd edn. Academic, San Diego
Khaled KF, Hamed MNH, Abdel-Azim KM, Abdelshafi NS (2011) Inhibition of copper corrosion in 3.5 % NaCl solutions by a new pyrimidine derivative: electrochemical and computer simulation techniques. J Solid State Electrochem 15:663–673
Muthukrishnan P, Jeyaprabha B, Prakash P (2013) Corrosion inhibition of Leucaena leucocephala pod on mild steel in sulphuric acid solution. Acta Metall Sin (Engl Lett) 26:416–424
Musa AY, Kadhum AAH, Mohamad AB, Takriff MS (2010) Experimental and theoretical study on the inhibition performance of triazole compounds for mild steel corrosion. Corros Sci 52:3331–3340
Li X, Deng S (2012) Inhibition effect of Dendrocalamus brandisii leaves extract on aluminium in HCl, H3PO4 solutions. Corros Sci 65:299–308
Singh A, Singh VK, Quraishi MA (2013) Inhibition of mild steel corrosion in HCl solution using Pipali (Piper longum) fruit extract. Arab J Sci Eng 38:85–97
Umoren SA, Li Y, Wang FH (2010) Synergistic effect of iodide ion and polyacrylic acid on corrosion inhibition of iron in H2SO4 investigated by electrochemical techniques. Corros Sci 52:2422–2429
Umoren SA, Solomon MM, Eduok UM, Obot IB, Israel AU (2014) Inhibition of mild steel corrosion in H2SO4 solution by coconut coir dust extract obtained from different solvent systems and synergistic effect of iodide ions: ethanol and acetone extracts. J Environ Chem Eng 2:1048–1060
Negma NA, Kandile NG, Badr EA, Mohammed MA (2012) Gravimetric and electrochemical evaluation of environmentally friendly nonionic corrosion inhibitors for carbon steel in 1 M HCl. Corros Sci 65:94–103
Tan YJ, Bailey S, Kinsellal B (1996) An investigation of the formation and destruction of corrosion inhibitor films using electrochemical impedance spectroscopy (EIS). Corros Sci 38:1545–1549
Sakunthala P, Vivekananthan SS, Gopiraman M, Sulochana N, Vincent AR (2013) Spectroscopic investigations of physicochemical interactions on mild steel in an acidic medium by environmentally friendly green inhibitors. J Surfact Deterg 16:251–263
Lebrini M, Robert F, Lecante A, Roos C (2011) Corrosion inhibition of C38 steel in 1 M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant. Corros Sci 53:687–695
Bentiss F, Jama C, Mernari B, El Attari H, El Kadi L, Lebrini M, Traisnel M, Lagrenée M (2009) Corrosion control of mild steel using 3,5-bis(4-methoxyphenyl)-4-amino-1,2,4-triazole in normal hydrochloric acid medium. Corros Sci 51:1628–1635
Yoo SH, Kim YW, Chung K, Kim NK, Kim JS (2013) Corrosion inhibition properties of triazine derivatives containing carboxylic acid and amine groups in 1.0 M HCl solution. Ind Eng Chem Res 52:10880–10889
Solmaz R (2014) Investigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-dimethylaminobenzylidene) rhodanine. Corros Sci 79:169–176
El-Sukkary MM, Soliman EA, El-Awady MY, Shaban SM (2013) Inhibition of mild steel corrosion in acidic medium by some cationic surfactants. J Ind Eng Chem 20:3524–3535
Yu Y, Zhuang YY, Wang ZH (2001) Adsorption of water-soluble dye onto functionalized resin. J Colloid Interf Sci 242:288–293
Umoren SA, Obot IB, Israel AU, Asuquo PO, Eduok UM, Solomon MM, Udoh AP (2014) Inhibition of mild steel corrosion in acidic medium using coconut coir dust extracted from water and methanol as solvents. J Ind Eng Chem 20:3612–3622
Noor EA (2011) The impact of some factors on the inhibitory action of Radish seeds aqueous extract for mild steel corrosion in 1 M H2SO4 solution. Mater Chem Phys 131:160–169
Obot IB, Umoren SA, Obi-Egbedi NO (2011) Corrosion inhibition and adsorption behavior for aluminium by extract of Aningeria robusta in HCl solution: synergistic effect of iodide ions. J Mater Environ Sci 2:60–71
Avci G, Keles Y (2011) Aqueous extract of Acacia cyanophylla leaves as environmentally friendly inhibitor for mild steel corrosion in 1 M H2SO4 solution. Surf Interface Anal 43:1311–1317
Obot IB, Obi-Egbedi NO, Umoren SA (2009) Antifungal drugs as corrosion inhibitors for aluminium in 0.1 M HCl. Corros Sci 51:1868–1875
Obot IB, Obi-Egbedi NO (2010) Adsorption properties and inhibition of mild steel in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros Sci 52:198–204
Obot IB, Obi-Egbedi NO, El-Khaiary MI (2011) Quantum chemical investigation and statistical analysis of the relationship between corrosion inhibition efficiency and molecular structure of xanthene and its derivatives on mild steel in sulphuric acid. J Mol Struct 1002:86–96
Ebenso EE, Kabanda MM, Arslan T, Saracoglu M, Kandemirli F, Murulanal LC, Singh AK, Shukla SK, Hammouti B, Khaled KF, Quraishi MA, Obot IB, Eddy NO (2012) Quantum chemical investigations on quinoline derivatives as effective corrosion inhibitors for mild steel in acidic medium. Int J Electrochem Sci 7:5643–5676
Kabanda MM, Murulana LC, Ozcan M, Karadag F, Dehri I, Obot IB, Ebenso EE (2012) Quantum chemical studies on the corrosion inhibition of mild steel by some triazoles and benzimidazole derivatives in acidic medium. Int J Electrochem Sci 7:5035–5056
Obot IB, Obi-Egbedi NO, Eseola AO (2011) Anticorrosion potential of 2-mesityl-1H-imidazo[4,5-f][1,10]-phenanthroline on mild steel in sulfuric acid solution: experimental and theoretical study. Ind Eng Chem Res 50:2098–2110
Kabanda MM, Obot IB, Ebenso EE (2013) Computational study of some amino acid derivatives as potential corrosion inhibitors for different metal surfaces and in different media. Int J Electrochem Sci 8:10839–10850
Awad MK, Mustafa MR, Abo Elnga MM (2010) Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface. J Mol Struct (THEOCHEM) 959:66–74
Zheng X, Zhang S, Li W, Yin L, He J, Wu J (2014) Investigation of 1-butyl-3-methyl-1H-benzimidazolium iodide as inhibitor for mild steel in sulfuric acid solution. Corros Sci 80:383–392
Umoren SA, Obot IB, Gasem ZM, Odewunmi NA (2014) Experimental and theoretical studies of red apple fruit extract as green corrosion inhibitor for mild steel in HCl solution. J Dispers Sci Technol. doi:10.1080/01932691.2014.922887
Negm NA, Lkholy YM, Zahran MK, Tawfik SM (2010) Corrosion inhibition efficiency and surface activity of benzothiazol-3-ium cationic Schiff base derivatives in hydrochloric acid. Corros Sci 52:3523–3536
Musa AY, Jalgham RTT, Mohamad AB (2012) Molecular dynamic and quantum chemical calculations for phthalazine derivatives as corrosion inhibitors of mild steel in 1 M HCl. Corros Sci 56:176–183
Obot IB, Obi-Egbedi NO, Ebenso EE, Afolabi AS, Oguzie EE (2012) Experimental, quantum chemical calculations, and molecular dynamic simulations insight into the corrosion inhibition properties of 2-(6-methylpyridin-2-yl)oxazolo[5,4 f][1,10]phenanthroline on mild steel. Res Chem Intermed 39:1927–1948
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umoren, S.A., Obot, I.B. & Gasem, Z.M. Adsorption and corrosion inhibition characteristics of strawberry fruit extract at steel/acids interfaces: experimental and theoretical approaches. Ionics 21, 1171–1186 (2015). https://doi.org/10.1007/s11581-014-1280-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1280-3