Abstract
In this study, chemically and electrochemically derived graphenes (CG and EG) were synthesized to form manganese dioxide (α-MnO2)/graphene nanocomposite cathode material for zinc primary cell. The discharge capacity of prepared nanocomposites was studied using Swagelok-type cell setup, where α-MnO2/graphene was used as a cathode and zinc as an anode along with an electrolyte (ZnCl2). Electrical conductivity and discharge behaviors of the CG and EG samples were studied in detail. The EG sample showed a high discharge capacity of 337 mAh g−1, whereas the CG sample showed a discharge capacity of 205 mAh g −1. More importantly, the discharge capacity of the EG sample-based cathode was 64 % higher than that of the CG sample-based cathode. The above observation suggests that EG sample serves as an effective cathode material for zinc-based primary battery applications.








Similar content being viewed by others
References
Vivekchand SRC, Rout CS, Subramanyam KS, Govindaraj A, Rao CNR (2008) Graphene-based electrochemical super capacitors. J Chem Sci 120:9–13
Wu H, Liu J, Aksay IA, Lin Y (2010) Graphene based electro chemical sensors and biosensors. Electro Analy 22(10):1027–36
Shinde DB, Debgupta J, Kushwaha A, Aslam M, Pillai VK (2011) Electrochemical unzipping of multi-walled carbon nanotubes for facile synthesis of high-quality graphene nanoribbons. J Ame Chem Soci 133:4168
Yoo EJ, Kim J, Hosono E, Zhou HS, Kudo T, Honma I (2008) Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett 8:2277–82
Wang C, Li D, Too CO, Wallace GG (2009) Electrochemical properties of graphene paper electrodes used in lithium batteries. Chem Mater 21(13):2604–06
Sima M, Enculescu I, Sima A (2011) Preparation of graphene and its application in dye-sensitized solar cells. Optoelect Adv Mater 5:414–18
Seger B, Kamat PV (2009) Electro catalytically active graphene–platinum nano composite. Role of 2-D carbon support in PEM fuel cells. J Phys Chem C 113(19):7990–95
Wu JB, Becerril HA, Bao ZN, Liu ZF, Chen YS, Peumans P (2008) Organic solar cells with solution-processed graphene transparent electrodes. Appl Phys Lett 92:263302–304
Dong LX, Chen Q (2010) Properties, synthesis, and characterization of graphene. Mater Sci 4:45–54
Tang L, Wang Y, Li Y, Feng H, Lu J, Li J (2009) Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv Func Mater 19:2782–89
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV (2004) Electric field effect in atomically thin carbon films. Science 306:666–9
Eda G, Fanchini G, Chhowalla M (2008) Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nature Nanotech 3:270–27
Simya OK, Selvam M, Karthik A, Rajendran V (2014) Dye sensitized solar cells (DSSC) based on visible light active TiO2 heterojunction nanoparticles. Synthetic Metal 188:124–129
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nature Nanotech 4:217–24
Wang G, Wang B, Park J, Wang, Sun YB, Yao J (2009) High efficient and large-scale synthesis of graphene by electrolytic exfoliation. Carbon 473:242–46
Luo Z, Lu Y, Somers LA, Johnson AT (2009) High yield preparation of macroscopic graphene oxide membranes. J Ame Chem Soc 131(3):898–9
O’Brien M, Nichols B (2010) CVD synthesis and characterization of graphene thin films. Army research laboratory ARL-TR- 5047
Reina A, Jia XT, Ho LY (2009) Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett 9(1):30–35
Reina A, Jia XT, Ho J, Nezich D, Son H, Bulovic V, Dresselhaus MS, Kong J (2009) Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett 9(5):1752–1758
Choucair M, Thordarson P, Stride JA (2009) Gram-scale production of graphene based on solvothermal synthesis and sonication. Nature Nanotech 4:30–33
Singh DK, Iyery PK, Giriz PK (2011) Improved chemical synthesis of graphene using a safer solvothermal route. Inter J Nano Sci 10(1):1–4
Titelman GI, Gelman V, Bron S, Khalfin RL, Cohen Y, Bianco-Peled H (2005) Synthesis of water soluble graphene. Carbon 43:641–649
Liu N, Luo F, Wu H, Liu Y, Zhang C, Chen J (2008) One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv Func Mater 18:1518–25
Kaniyoor A, Baby T, Ramaprabhu S (2010) Graphene synthesis via hydrogen induced low temperature exfoliation of graphite oxide. J Mater Chem 20:8467–69
Selvam M, Sakthipandi K, Suryaprabha R, Saminathan K, Rajendran V (2013) Synthesis and characterisation of electrochemically-reduced graphene. Bulli Mater Sci 36(7):1315–1321
Su CY, Lu AU, Xu Y, Chen FR, Khlobystov AN, Li LJ (2011) High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 5(3):2332–2339
Gijie S, Han S, Wang M, Wang KL, Kaner RB (2007) A chemical route to graphene for device applications. Nano Lett 7:3394–3398
Stankovih S, Dikin DA, Dommett GHB, Rohlhaas KM, Zimney EJ, Stach EA (2006) Graphene based composite material. Nature 442:282–6
Wang JJ, Zhu MY, Outlaw RA, Zhao X, Manos DM, Holloway BC, Mammana VP (2004) Synthesis and field emission properties of carbon nanostructures. Appl Phys Lett 85:1265
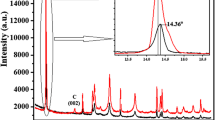
Ju HM, Choi SH, Huh SH (2010) X- ray diffraction patterns of thermally-reduced graphenes. J Korean Phys Soci 57:1649–52
Parthasarathy G, Sreedhar B, Chetty RK (2006) Spectroscopic and X-ray diffraction studies on fluid deposited rhombohedral graphite from the Eastern Ghats Mobile Belt. India Curr Sci 90(7):995–00
Ghosh D, Chandra S, Chakraborty A, Ghosh SK, Pramanik PA (2010) Novel graphene oxide para amino benzoic acid nanosheet as effective drug delivery system to treat drug resistance bacteria. Inter J Pharm Sci Drug Res 2(2):127–133
Ramesh GK, Sampath S (2009) Electrochemical reduction of oriented graphene oxide films: an in situ Raman spectro electrochemical study. J Phys Chem C 113(19):7985–89
Ferrari AC (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97:18
Ni Z, Wang Y, Yu T, Shen Z (2008) Raman spectroscopy and imaging of graphene. Nano Res 1:273–91
Krishnamurthy K, Veerapandian M, Mohan R, Kim SJ (2008) Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl Phys A MaterSci Proce 90:1
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous. Carbon Phys Rev B 61:14095–07
Schwab T, Burg BR, Schirmar NC, Poulikakos D (2009) An electrical method for the measurement of the thermal and electrical conductivity of reduced graphene oxide nanostructures. Nano Tech 20:405704
Sundar Pethaiah S, Arun Kumar J, Kalyani P (2011) Improvement in the discharge characteristics of zinc–carbon primary cells: a comparative study with various carbon additives. Ionics 17:339–342
Srither SR, Selvam M, Arunmetha S, Yuvakkumar R, Saminathan K, Rajendran V (2013) Enhancement of discharge capacity of Mg/MnO2 primary cell with nano-MnO2 as cathode. Sci Adv Mater 5:1–5
Garcia EM, Tarôco HA, Melo JOF, Silva APCM, Oliveira IMF (2013) Electrochemical recycling of Zn from spent Zn–MnO2 batteries. Ionics 19:1699–1703
Xu C, Li B, Du H, Kand F (2012) Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew Chem Int Ed 51:933–935
Kim KS, Zhao Y, Jang H (2009) Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457(7230):706–10
Acknowledgments
The author (M. Selvam) is thankful to the Department of Science and Technology (DST), New Delhi, for providing the Inspire fellowship (IF110749) to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selvam, M., Srither, S.R., Saminathan, K. et al. Chemically and electrochemically prepared graphene/MnO2 nanocomposite electrodes for zinc primary cells: a comparative study. Ionics 21, 791–799 (2015). https://doi.org/10.1007/s11581-014-1234-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1234-9