Abstract
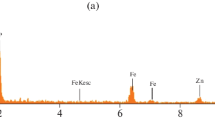
The structure of K0.92(2) Zn0.08(2) H1.92(2) (PO4) was determined using single-crystal X-ray diffraction. The crystal structure of the Zn-KDP belonged to the tetragonal space group \( \mathrm{I}\overline{4}2\mathrm{d} \), with cell parameters of a = b = 7.4487(5) Å and c = 6.9703(5) Å, 386.73(5) Å3, Z = 4, and R = 0.023. Zn2+ ions were used as substitutes for K+ ions with hydrogen vacancy. The Zn-KDP single crystals were submitted to further Raman, infrared, and 1H NMR studies to investigate chemical group functionalisation, possible bonding between the organic and inorganic materials, and partial substitution of K+ by Zn2+. The latter partial substitution was confirmed by the deviation of IR frequencies for O–H stretching, the variation of IR and Raman frequencies for stretching and bending vibrations ν(PO4) of H2PO4, and the appearance of additional Raman (147, 386 and 481 cm−1) vibrational bands. Electrical conductivity measurements were performed on polycrystalline pellets of Zn-KDP and pure KDP at room temperatures (RT) of up to 473K. In both cases, a conductivity jump close to 453K was observed, and a stronger increase of conductivity was measured.








Similar content being viewed by others
References
Ponomareva VG, Martsinkevich VV, Chesalov YA (2011) Transport and thermal characteristics of Cs1 – x Rb x H2PO4 1. J Electrochem 47:645–653
Botez CE, Carbajal D, Adiraju VAK, Tackett RJ, Chianelli RR (2010) Intermediate-temperature polymorphic phase transition in KH2PO4: a synchrotron X-ray diffraction study. J Phys Chem Solids 71:1576–1580
Subramony JA, Scott L, Kahr B (1998) Polymorphism of potassium dihydrogen phosphate. J Chem Mater 10:2053–2057
Anand Subramony J, Lovell S, Kaminsky W, Kahr B (2004) Structure of a high temperature phase of potassium dideuteriophosphate (DKDP). J Solid State Commun 132:827–830
Park J-H (2002) Separation of surface and bulk electrical conductivity in KH2PO4 and KH2AsO4 crystals at high temperatures. J Solid State Commun 123:291–294
Guzman LA, Maeda K, Hirota S, Kubota N (1998) Adsorption of growth suppressor chromium (III) on potassium sulfate crystals in aqueous solution. J Cryst Growth 186:438–445
Rashkovich LN, Kronsky NV (1997) Influence of of Fe 3+ and Al 3+ ions on the kinetics of steps on the faces of KDP. J Cryst Growth 182:434–441
Zaitseva N, Carman L, Smolsky I, Torres R, Yan M (1999) The effect of impurities and supersaturation on the rapid growth of KDP crystals. J Cryst Growth 204:512–524
Guzman LA, Maeda K, Hirota S, Yokota M, Kubota N (1997) Unsteady-state impurity effect of chromium (III) on the growth rate of potassium sulfate crystal in aqueous solution. J Cryst Growth 181:272–280
Lai X, Roberts KJ, Bedzyk MJ, Lyman PF, Cardoso LP, Sasaki JM (2005) Structure of habit-modifying trivalent transition metal cations (Mn3+, Cr3+) in nearly perfect single crystals of potassium dihydrogen phosphate as examined by X-ray standing waves, X-ray absorption spectroscopy, and molecular modeling. J Chem Mater 17:4053–4061
Garces NY, Stevens KT, Halliburton LE, Yan M, Zaitseva NP, DeYoreo JJ (2001) Optical absorption and electron paramagnetic resonance of Feions in KDP crystals. J Cryst Growth 225:435–439
Asakuma Y, Takeda S, Maeda K, Fukui K (2009) Kinetic and theoretical studies of metal ion adsorption in KDP solution. J Appl Surf Sci 225:4140–4144
Eremina TA, Kuznetsov VA, Eremin NN, Okhrimenko TM, Furmanova NG, Efremovaa EP, Rak M (2005) On the mechanism of impurity influence on growth kinetics and surface morphology of KDP crystals-II: experimental study of influence of bivalent and trivalent impurity ions on growth kinetics and surface morphology of KDP crystals. J Cryst Growth 273:586–593
Benedict JB, Wallace PM, Reid PJ, Jang S-H, Kahr B (2003) Up-conversion luminescence in dye doped crystals of potassium hydrogen phthalate. J Adv Mater 15:1068–1070
Pritula I, Gayvoronsky V, Gromov Y, Kopylovsky M, Kolybaeva M, Puzikov V, Kosinova A, Savvin Y, Velikhov Y, Levchenko A (2009) Linear and nonlinear optical properties of dye-doped KDP crystals: effect of thermal treatment. J Optics Commun 282:1141–1147
Asakuma Y, Nishimura M, Li Q, Ming Ang H, Tade M, Maeda K, Fukui K (2007) Colouring mechanism of dyed KDP crystal by quantum chemistry. J Mol Struct (THEOCHEM) 810:7–13
Zaitseva N, Carman L, Rapid growth of KDP-type crystals (2001) Rapid growth of KDP-type crystals. J Mater 43:1–118
Velikhov Y, Pritula I, Ganina I, Kolybayeva M, Puzikov V, Levchenko AN (2007) Growth and properties of dyed KDP crystals. J Cryst Res Technol 42:27–33
Nelmes RJ (1987) Ferroelectrics 71:87
Sheldrick GM (1997) SHELXTL-97 Program for Refining Crystal Structure, University of Göttingen. Göttingen, Germany
Kawahata Y, Tominaga Y (2008) Dynamical mechanism of ferroelectric phase transition in KDP/DKDP mixed crystals and distortion of PO4 tetrahedron. J Solid State Commun 145:218–222
Dongli X, Xue D, Ratajczak H (2005) Morphology and structure studies of KDP and ADP crystallites in the water and ethanol solutions. J Mol Struct 740:37–45
Kumaresan P, Moorthy Babu S, Anbarasan PM (2008) Growth and characterization of metal ions and dyes doped KDP single crystals for laser applications. J Mater Res Bull 43:1716–1723
Frost RLM, Bouzaid JM, Reddy BJ (2007) Vibrational spectroscopy of the sorosilicate mineral hemimorphite Zn4(OH)2Si2O7.H2O. J Polyhedron 26(12):2405–2412
Muralidharan P, Venkateswarlu M, Satyanarayana N (2005) Acid catalyst concentration effect on structure and ion relaxation studies of Li2O–O5–O3–SiO2 glasses synthesized by sol–gel process. J Non-Cryst Solid 351:583–594
O'Keefee M, Perrino CT (1967) Proton conductivity in pur and doped KH2PO4. J Phys Chem Solids 28:211–218
Mathew M, Wong-Ng W (1995) Crystal structure of a new monoclinic form of potassium dihydrogen phosphate containing orthophosphacidiumion, [H4PO4]+1. J Solid State Chem 114:219–223
Acknowledgments
The authors wish to express their gratitude to Mr. Anouar Smaoui and Mrs Hanen Ben Salem from the Faculty of Science, Sfax, Tunisia for their constructive proofreading and language polishing services. Thanks are also due to the Raman spectroscopy Unit at FSS Sfax for their valuable help with the measuring of Raman spectra.
Author information
Authors and Affiliations
Corresponding author
Appendix A. Supplementary data
Appendix A. Supplementary data
CSD-423866 contains the supplementary crystallographic data for this paper. CIF and structure factor deposits may be made using online service: http://journals.iucr.org/services/cif/checkcif.html, or from the Fachinformationszentrum Karlsruhe (FIZ) 76344 Eggenstein-Leopoldshafen, Germany; tel: +49-(0)7247/808-205; or e-mail: crysdata@fiz-karlsruhe.de
Rights and permissions
About this article
Cite this article
Ettoumi, H., Brahim, F.B., Toumi, M. et al. Synthesis, crystal structure, and spectroscopic study of K0.92(2) Zn0.08(2) H1.92(2) (PO4) (Zn-KDP). Ionics 19, 193–200 (2013). https://doi.org/10.1007/s11581-012-0824-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0824-7