Abstract
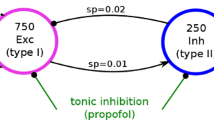
The neuronal mechanisms of general anesthesia are still poorly understood. Besides several characteristic features of anesthesia observed in experiments, a prominent effect is the bi-phasic change of power in the observed electroencephalogram (EEG), i.e. the initial increase and subsequent decrease of the EEG-power in several frequency bands while increasing the concentration of the anaesthetic agent. The present work aims to derive analytical conditions for this bi-phasic spectral behavior by the study of a neural population model. This model describes mathematically the effective membrane potential and involves excitatory and inhibitory synapses, excitatory and inhibitory cells, nonlocal spatial interactions and a finite axonal conduction speed. The work derives conditions for synaptic time constants based on experimental results and gives conditions on the resting state stability. Further the power spectrum of Local Field Potentials and EEG generated by the neural activity is derived analytically and allow for the detailed study of bi-spectral power changes. We find bi-phasic power changes both in monostable and bistable system regime, affirming the omnipresence of bi-spectral power changes in anesthesia. Further the work gives conditions for the strong increase of power in the δ-frequency band for large propofol concentrations as observed in experiments.












Similar content being viewed by others
References
Agmon-Sir H, Segev I (1993) Signal delay and input synchronisation in passive dendritic structures. J Neurophysiol 70:2066–2085
Alkire M, Hudetz A, Tononi G (2008) Consciousness and anesthesia. Science 322:876–880
Amari S (1977) Dynamics of pattern formation in lateral-inhibition type neural fields. Biol Cybern 27:77–87
Amit DJ (1989) Modeling brain function: the world of attactor neural networks. Cambridge University Press, Cambridge
Andrews D, Leslie K, Sessler D, Bjorksten A (1997) The arterial blood propofol concentration preventing movement in 50 of healthy women after skin incision. Anesth Analg 85:414–419
Antkowiak B (1999) Different actions of general anesthetics on the firing patterns of neocortical neurons mediated by the GABAA-receptor. Anesthesiology 91:500–511
Antkowiak B (2002) In vitro networks: cortical mechanisms of anaesthetic action. Br J Anaesth 89(1):102–111
Atay FM, Hutt A (2005) Stability and bifurcations in neural fields with finite propagation speed and general connectivity. SIAM J Appl Math 65(2):644–666
Atay FM, Hutt A (2006) Neural fields with distributed transmission speeds and constant feedback delays. SIAM J Appl Dyn Syst 5(4):670–698
Bai D, Pennefather P, MacDonald J, Orser BA (1999) The general anesthetic propofol slows deactivation and desensitization of GABAA receptors. J Neurosci 19(24):10635–10646
Baker PM, Pennefather PS, Orser BA, Skinner F (2002) Disruption of coherent oscillations in inhibitory networks with anesthetics: role of GABA-A receptor desensitization. J. Neurophysiol 88:2821–2833
Bojak I, Liley D (2005) Modeling the effects of anesthesia on the electroencephalogram. Phys Rev E 71:041902
Braitenberg V, Schütz A (1998) Cortex : statistics and geometry of neuronal connectivity, 2nd edn. Springer, Berlin
Bressloff PC (2001) Traveling fronts and wave propagation failure in an inhomogeneous neural network. Physica D 155:83–100
Bressloff PC, Cowan JD, Golubitsky M, Thomas PJ, Wiener MC (2002) What geometric visual hallucinations tell us about the visual cortex. Neural Comput 14:473–491
Carstens E, Antognini J (2005) Anesthetic effects on the thalamus, reticular formation and related systems. Thal Rel Syst 3:1–7
Chacron MJ, Longtin A, Maler L (2005) Delayed excitatory and inhibitory feedback shape neural information transmission. Phys Rev E 72:051917
Coombes S (2005) Waves, bumps and patterns in neural field theories. Biol Cybern 93:91–108
Coombes S, Lord G, Owen M (2003) Waves and bumps in neuronal networks with axo-dendritic synaptic interactions. Physica D 178:219–241
Coombes S, Venkov N, Shiau L, Bojak I, Liley D, Laing C (2007) Modeling electrocortical activity through improved local approximations of integral neural field equations. Phys Rev E 76:051901–0519018
de Jong R, Eger E (1975) Mac expanded: AD50 and AD95 values of common inhalation anesthetics in man. Anesthesiol 42(4):384–389
Destexhe A, Contreras D (2006) Neuronal computations with stochastic network states. Science 314:85–90
Dutta S, Matsumoto Y, Gothgen N, Ebling W (1997) Concentration-EEG effect relationship of propofol in rats. J Pharm Sci 86(1):37
Eggert J, van Hemmen JL (2001) Modeling neuronal assemblies: theory and implementation. Neural Comput 13(9):1923–1974
Fell J, Widman G, Rehberg B, Elger C, Fernandez G (2005) Human mediotemporal EEG characteristics during propofol anesthesia. Biol Cybern 92:92–100
Forrest F, Tooley M, Saunders P, Prys-Roberts C (1994) Propofol infusion and the suppression of consciousness: the EEG and dose requirements. Br J Anaesth 72:35–41
Foster B, Bojak I, Liley DJ (2008) Population based models of cortical drug response: insights from anaesthesia. Cogn Neurodyn 2:283–296
Franks N (2008) General anesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9:370–386
Franks N, Lieb W (1994) Molecular and cellular mechanisms of general anesthesia. Nature 367:607–614
Freeman W (1979) Nonlinear gain mediating cortical stimulus-response relations. Biol Cybern 33:237–247
Freeman W (1992) Tutorial on neurobiology: from single neurons to brain chaos. Int J Bifurcat Chaos 2(3):451–482
Gammaitoni L, Hanggi P, Jung P (1998) Stochastic resonance. Rev Modern Phys 70(1):223–287
Gerstner W, Kistler W (2002) Spiking neuron models. Cambridge University Press, Cambridge
Han T, Lee J, Kwak I, Kil H, Han K, Kim K (2005) The relationship between bispectral index and targeted propofol concentration is biphasic in patients with major burns. Acta Anaesthesiol Scand 49:85–91
Hellwig B (2000) A quantitative analysis of the local connectivity between pyramidal neurons in layers 2/3 of the rat visual cortex. Biol Cybern 82:11–121
Hemmings Jr. H, Akabas M, Goldstein P, Trudell J, Orser B, Harrison N (2005) Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci 26(10): 503–510
Hutt A (2007) Generalization of the reaction-diffusion, Swift–Hohenberg, and Kuramoto–Sivashinsky equations and effects of finite propagation speeds. Phys Rev E 75:026214
Hutt A (2008) Local excitation-lateral inhibition interaction yields oscillatory instabilities in nonlocally interacting systems involving finite propagation delay. Phys Lett A 372:541–546
Hutt A, Atay F (2005) Analysis of nonlocal neural fields for both general and gamma-distributed connectivities. Physica D 203:30–54
Hutt A, Atay F (2006) Effects of distributed transmission speeds on propagating activity in neural populations. Phys Rev E 73:021906
Hutt A, Atay F (2007) Spontaneous and evoked activity in extended neural populations with gamma-distributed spatial interactions and transmission delay. Chaos Solitons Fractals 32:547–560
Hutt A, Frank T (2005) Critical fluctuations and 1/f -activity of neural fields involving transmission delays. Acta Phys Pol A 108(6):1021
Hutt A, Schimansky-Geier L (2008) Anesthetic-induced transitions by propofol modeled by nonlocal neural populations involving two neuron types. J Biol Phys 34(3–4):433–440
Hutt A, Bestehorn M, Wennekers T (2003) Pattern formation in intracortical neuronal fields. Network Comput Neural Syst 14:351–368
Hutt A, Sutherland C, Longtin A (2008) Driving neural oscillations with correlated spatial input and topographic feedback. Phys Rev E 78:021911
John E, Prichep L (2005) The anesthetic cascade: a theory of how anesthesia suppresses consciousness. Anesthesiol 102:447–471
Kaisti K, Metshonkala L, Ters M, Oikonen V, Aalto S, Jskelinen S, Hinkka S, Scheinin H (2002) Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiol 96:1358–1370
Kazama T, Ikeda K, Morita K, Sanjo Y (1998) Awakening propofol concentration with and without blood-effect site equilibration after short-term and long-term administration of propofol and fentanyl anesthesia. Anesthesiology 88(4):928–934
Kitamura A, Marszalec W, Yeh J, Narahashi T (2002) Effects of halothane and propofol on excitatory and inhibitory synaptic transmission in rat cortical neurons. J Pharmacol 304(1):162–171
Koch C (1999) Biophysics of computation. Oxford University Press, Oxford
Kuizenga K, Kalkman C, Hennis PJ (1998) Quantitative electroencephalographic analysis of the biphasic concentration-effect relationship of propofol in surgical patients during extradural analgesia. Br J Anaesth 80:725–732
Kuizenga K, Wierda J, Kalkman C (2001) Biphasic EEG changes in relation to loss of consciousness during induction with thiopental, propofol, etomidate, midazolam or sevoflurane. Br J Anaesth 86(3):354–360
Laing C, Troy W (2003) PDE methods for non-local models. SIAM J Appl Dyn Syst 2(3):487–516
Liley D, Bojak I (2005) Understanding the transition to seizure by modeling the epileptiform activity of general anaesthetic agents. J Clin Neurophysiol 22:300–313
Liley D, Cadusch P, Wright J (1999) A continuum theory of electrocortical activity. Neurocomputing 26-27:795–800
Liu G (2004) Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci 7:373–379
Marik P (2004) Propofol: therapeutic indications and side-effects. Curr Pharm Des 10(29):3639–3649
Masuda N, Doiron B, Longtin A, Aihara K (2005) Coding of temporally varying signals in networks of spiking neurons with global delayed feedback. Neural Comput 17:2139–2175
McKernan M, Rosendahl T, Reynolds D, Sur C, Wafford K, Atack J, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore K, Carling R, Street L, Castro J, Ragan C, Dawson G, Whiting P (1997) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor A1 subtype. Nat Neurosci 3(6):587–592
Megias M, Emri Z, Freund T, Gulyas A (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102:527–540
Mell B, Schiller J (2004) On the fight between excitation and inhibition: location is everyting. Sci STKE, p. 44
Molaee-Ardekani B, Senhadji L, Shamsollahi M, Vosoughi-Vahdat B, Wodey E (2007) Brain activity modeling in general anesthesia: enhancing local mean-field models using a slow adaptive firing rate. Phys Rev E 76:041911
Musizza B, Stefanovska A, McClintock P, Palus M, Petrovcic J, Ribaric S, Bajrovic F (2007) Interactions between cardiac, respiratory and EEG-delta oscillations in rats during anaesthesia. J Physiol Lond 580:315–326
Mustola S, Baer G, Toivonen J, Salomaki A, Scheinin M, Huhtala H, Laippala P, Jantti V (2003) Electroencephalographic burst suppression versus loss of reflexes anesthesia with propofol or thiopental: differences of variance in the catecholamine and cardiovascular response to tracheal intubation. Anesth Analg 97:1040–1045
Nicholson C, Freeman J (1975) Theory of current source-density analysis and determination of conductivity tensor for anuran cerebellum. J Neurophysiol 38:356–368
Nunez P (1974) The brain wave equation: a model for the EEG. Math Biosci 21:279–291
Nunez P (1981) Electrical fields of the brain. Oxford University Press, Oxford
Nunez P (1995) Neocortical dynamics and human EEG rhythms. Oxford University Press, New York
Nunez P (2000) Toward a quantitative description of large-scale neocortical dynamic function and EEG. Behav Brain Sci 23:371–437
Nunez P, Srinivasan R (2006) Electric fields of the brain: the neurophysics of EEG. Oxford University Press, New York
Orser B (2007) Lifting the fog around anesthesia. Sci Am 7:54–61
Otsuka T, Kawaguchi Y (2009) Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. J Neurosc 29(34):10533–10540
Pittson S, Himmel A, MacIver M (2004) Multiple synaptic and membrane sites of anesthetic action in the ca1 region of rat hippocampal slices. BMC Neurosci 5:52
Rampil I, King B (1996) Volatile anesthetics depress spinal motor neurons. Anesthesiology 85:129–134
Rennie C, Robinson P, Wright J (2002) Unified neurophysical model of EEG spectra and evoked potentials. Biol Cybern 86:457–471
Robinson P (2003) Neurophysical theory of coherence and correlations of electroencephalographic and electrocorticographic signals. J Theor Biol 222:163–175
Robinson P, Loxley P, O’Connor S, Rennie C (2001) Modal analysis of corticothalamic dynamics, electroencephalographic spectra and evoked potentials. Phys Rev E 63:041909
Robinson P, Whitehouse R, Rennie C (2003) Nonuniform corticothalamic continuum model of encephalographic spectra with application to split-alpha peaks. Phys Rev E 68:021922
Robinson P, Rennie CJ, Rowe DL, O’Connor SC (2004) Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Hum Brain Mapp 23:53–72
Rundshagen I, Schroeder T, Prochep I, John E, Kox W (2004) Changes in cortical electrical activity during induction of anaesthesia with thiopental/fentanyl and tracheal intubation: a quantitative electroencephalographic analysis. Br J Anaesth 92(1):33–38
Smetters D (1995) Electrotonic structure and synaptic integration in cortical neurons. Ph.D. thesis, Massachusetts Institute of Technology, Cambridge, Massachusetts
Srinivasan R, Nunez P, Silberstein R (1998) Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Trans Biomed Eng 45:814–827
Steyn-Ross M, Steyn-Ross D (1999) Theoretical electroencephalogram stationary spectrum for a white-noise-driven cortex: evidence for a general anesthetic-induced phase transition. Phys Rev E 60(6):7299–7311
Steyn-Ross M, Steyn-Ross D, Sleigh J, Wilcocks L (2001a) Toward a theory of the general-anesthetic-induced phase transition of the cerebral cortex: I. A thermodynamic analogy. Phys Rev E 64:011917J
Steyn-Ross M, Steyn-Ross D, Sleigh J, Wilcocks L (2001b) Toward a theory of the general-anesthetic-induced phase transition of the cerebral cortex: II. Numerical simulations, spectra entropy, and correlation times. Phys Rev E 64:011918
Steyn-Ross M, Steyn-Ross D, Sleigh J (2004) Modelling general anaesthesia as a first-order phase transition in the cortex. Prog Biophys Mol Biol 85(2-3):369–385
Stienen P, van Oostrom H, Hellebrekers L (2008) Unexpected awakening from anaesthesia after hyperstimulation of the medial thalamus in the rat. Brit J Anaesth 100(6):857–859
van Hemmen J (2004) Continuum limit of discrete neuronal structures: is cortical tissue an ‘excitable’ medium? Biol Cybern 91(6):347–358
Venkov N, Coombes S, Matthews P (2007) Dynamic instabilities in scalar neural field equations with space-dependent delays. Physica D 232:1–15
Veselis R, Reinsel R, Beattie B, Mawlawi O, Feschenko V, DiResta G, Larson S, Blasberg R (1997) Midazolam changes cerebral bloodflow in discrete brain regions: an h2(15)o positron emission tomography study. Anesthesiol 87:1106–1117
Wessen A, Persson P, Nilsson A, Hartvig P (1993) Concentration-effect relationships of propofol after total intravenous anesthesia. Anesth Analg 77:1000–1007
Wilson M, Sleigh J, Steyn-Ross A, Steyn-Ross M (2006) General anesthetic-induced seizures can be explained by a mean-field model of cortical dynamics. Anesthsiol 104(3):588–593
Wright J, Kydd R (1992) The electroencephaloggram and cortical neural networks. Network 3:341–362
Wright J, Liley D (1995) Simulation of electrocortical waves. Biol Cybern 72:347–356
Wright J, Liley D (2001) A millimetric-scale simulation of electrocortical wave dynamics based on anatomical estimates of cortical synaptic density. Biosystems 63:15–20
Yang C, Shyr M, Kuo T, Tan P, Chan S (1995) Effects of propofol on nociceptive response and power spectra of electroencephalographic and sytemic arterial pressure signals in the rat: correlation with plasma concentration. J Pharmacol Exp Ther 275:1568–1574
Ying S, Goldstein P (2005) Propofol-block of SK channels in reticular thalamic neurons enhances gabaergic inhibition in relay neurons. J Neurophysiol 93:1935–1948
Yoshioka T, Levitt J, Lund J (1992) Intrinsic lattice connections of macaque monkey visual cortical area v4. J Neurosci 12(7):2785–2802
Acknowledgment
The authors acknowledge the financial support of NSERC Canada.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Variables from section "Linear stability"
This section gives the polynomial constants of the characteristic Eq. 27:
with
and τ e = σ e /v,τ i = σ i /v. For p = 1, δ E , δ I ≈ 0 and A 0, B 0, A 1, B 1 ≈ 0. Hence the pre-factors of the polynom (27) read
The autocorrelation function
To obtain the effective membrane potential u(x,t) in section "The general power spectrum", we may write
Here G(x,t) is the Greens’ function of the system, \(\tilde{G}(k,\omega)\) denotes its Fourier transform and \(\tilde{\Upgamma}(k,\omega)\) is the Fourier transform of the external stimulus Γ(x,t). Considering (53), then the correlation function reads
Since the power spectrum p LFP (x,ω) is defined by C LFP (x,τ), we deduce from (54) that the power spectrum is determined by the Fourier transform of the Greens’ function \(\tilde{G}(k,\omega)\) and the input correlation function in Fourier space \(\langle \tilde{\Upgamma}(k,\omega)\tilde{\Upgamma}(k^\prime,\omega^\prime)\rangle.\)
The Greens’ function
To compute the Greens function, we apply the Fourier transform in space to Eqs. 33 and 34, and obtain
Then it follows that
with
In addition
is the spatial Fourier transform of G(x,t) and we write \(\tilde{u}(k,t)\) using (52) as
Further we recall the identity (see e.g. Atay and Hutt 2006)
and obtain from (55), (56), (57) and (58) after a Fourier transformation into frequency space
with
and the kernel Fourier moments (Atay and Hutt 2005)
The external input
Considering the input (36), then the Fourier transform in space and time yields
with the Fourier transform of the external signal \(\tilde{\xi}(k,\omega).\) Since the external fluctuations in Fourier space are uncorrelated,
we obtain finally
dX/dp > 0 in single stationary solutions
Considering Eq. 47,
with f′ = df/dp > 0, δ′ = ∂δ/∂ρ > and ρ′ = dρ/dp > 0. Further Fig. 3b illustrates the limits \(\bar{V}_-\gg\Uptheta (\rho\approx 0)\) for p ≈ 1 and \(\bar{V}_-\to\Uptheta (\rho \to 1)\) for p → ∞ and section "The resting state" shows that a e − a i f < 0. Then (65) gives \({\frac{dX(p)}{dp}} > 0\) for all p.
The power spectrum for large frequencies
To compute p EEG(ν), we consider Eq. 41 and compute the Greens’ function (59) in the long wavelength limit as
with \({\mathcal L}_0(p,\nu), {\mathcal L}_1(p,\nu)\) defined as
with
and
Equation 66 assumes the approximation of a large but finite propagation speed v. Then inserting (66) into (41) yields the power spectrum
The functions \({\mathcal L}_{1,r}, {\mathcal L}_{1,i}\) depend on the propagation speed and are small compared to \({\mathcal L}_{0,r}.\) Hence neglecting terms containing \({\mathcal L}_{1,r}, {\mathcal L}_{1,i},\) we find the conditions (50).
Rights and permissions
About this article
Cite this article
Hutt, A., Longtin, A. Effects of the anesthetic agent propofol on neural populations. Cogn Neurodyn 4, 37–59 (2010). https://doi.org/10.1007/s11571-009-9092-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-009-9092-2