Abstract
Background
Hydrophilic polymers have been shown to improve physiologic recovery following repair of transected nerves with microsutures. Our study was designed to combine hydrophilic polymer therapy with nerve tubes (NT) to enhance polymer delivery to the site of nerve injury.
Methods
Using a rat sciatic nerve injury model, a single transection injury was repaired in an end-to-end fashion with NT + polyethylene glycol (PEG) to NT alone. Compound action potentials (CAPs) were recorded before nerve transection and after repair. Behavioral testing was performed for 5 weeks.
Results
PEG therapy restored CAPS in all, but one, animals, while no CAPS were recorded in animals not receiving PEG. Behavioral nerve function was measured using the standardized functional assessment technique and foot fault asymmetry scores (FF). FF scores were improved for the PEG therapy groups on postoperative days 7, 14, and 21. However, after expected eventual axonal outgrowth, the benefit was less noticeable at days 28 and 35. Immunohistochemistry of the distal axon segments showed an increase number of sensory and motor axons in the NT + PEG group as compared to NT alone.
Conclusion
These data suggest that PEG delivery via a conduit may provide a simple, effective way to fuse severed axons and regain early nerve function. For proximal nerve injuries in large animals, recovery of axonal continuity could dramatically improve outcomes, even if fusion only occurs in a small percentage of axons.
Similar content being viewed by others
Introduction
Current strategies for peripheral nerve repair are limited. With no best treatment option, techniques such as nerve tubes, nerve grafts, tissue matrices, and nerve growth guides have been designed to enhance the number of regenerating axons [8]. Unfortunately, using these techniques, regenerating axons require months to reach denervated target tissues when injuries are proximally located [8, 11, 31]. This inability to rapidly restore the loss of physiological function that results from axonal injury continues to produce poor clinical outcomes [8].
Invertebrate animals exhibit a rapid and highly specific neuronal recovery by an innate mechanism known as axonal fusion [10, 24]. Recent attempts at fusion using hydrophilic polymers, such as polyethylene glycol (PEG), have also been successful at improving physiologic and behavioral recovery after traumatic peripheral neuropathy in mammalian organisms [4, 6, 19, 29]. In cases of nerve transection, PEG therapy has also been shown to improve outcomes when combined with microsuture repair [4, 29]. Microsutures, however, may lead to increased scarring and decreased electrical conductance at the site of injury compared to alternative methods of coaptation [13].
Nerve conduits provide a semipermeable framework allowing for resorption and diffusion [18, 32]. Available examples can be collagen or made using xenograft tissue such as small bowel mucosa. In animal studies, these conduits have shown similar long-term physiologic recovery compared to autografts [2, 18]. In this study, we hypothesized that using PEG fusion could be combined with nerve tubes and would lead to improved outcomes compared to animals that received nerve tube repair alone.
Methods
All experimental procedures were approved by and performed in accordance with the standards set forth by the Institutional Animal Care and Use Committee at Vanderbilt University.
Surgical Procedures
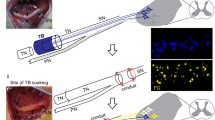
Female Sprague–Dawley rats were anesthetized with inhaled isoflurane and the left hindlimb shaved and aseptically prepped. A 2-cm incision was made parallel to the femur, and, using sharp dissection, the biceps femoris muscle was split to expose the left sciatic nerve. The exposed nerve was bathed in Plasma-lyte A® (Baxter; Deerfield, IL) (in mEq/L, Na 140, K 5, Mg 3, Cl 98, acetate 27, gluconate 23; pH 7.4, 294 mOsm/L), and electrophysiological testing was performed.
For the two groups, nerve tubes (NT) + polyethylene glycol (PEG) and NT alone, the sciatic nerve was then completely transected at the midpoint and the site was irrigated with Plasma-lyte A®. In each group, the nerve ends were then brought together, in apposition, inside a commercially available type 1 collagen nerve tube (NeuroMatrix, Stryker; Collagen Matrix, Inc., Franklin Lakes, NJ). Using standard microsurgical techniques, the distal ends of the tube were sutured to the epineurium using two 9-0 Ethilon (Ethicon, Somerville, NJ). Once the nerve was repaired, a 1 % solution of MB (Acros Organics; Morris Plains, NJ) in sterile water was applied to the site of nerve transection through a small slit in the nerve tube. A 50 % by weight solution of PEG (3.35 kD molecular weight, Sigma-Aldrich; St. Louis, MO) in sterile water was then applied to the same site for 1 min in the NT + PEG group, while the nerve tube control (NT alone) animals received a final solution containing only sterile water. Randomization was accomplished by having two syringes labeled with a one or two. The surgical operator was unaware of the contents of the syringe because it was prepared by a separate individual. The animals were marked in numerical order as NT#. Rats were identified only by number until all the data was collected, only then were the rats’ identity by number and experimental group known.
The nerve transection site and wound bed were then irrigated with Lactated Ringers (Hospira; Lake Forest, IL) (in mEq/L, Na 130, K 4, Ca 2.7, Cl 109, and lactate 28; pH 6.5, 273 mOsm/L), and electrophysiological testing was repeated. The skin was approximated using a running subcuticular 5-0 MONOCRYL suture (Ethicon, Somerville, NJ), and animals were allowed to recover from anesthesia. Postoperatively, all animals were then given a subcutaneous injection of ketoprofen (5 mg/kg). Ketoprofen is almost completely excreted by 24 h postoperatively [14].
Animals were divided into two groups: NT + polyethylene glycol (PEG) or NT alone (control). The NT + PEG group had nine rats, and the NT alone had seven rats. Compound action potentials (CAPs) were recorded before nerve transection and after solution therapy. After behavioral testing was completed, rats were then sacrificed via intracardiac injection of Fatal-Plus Solution (Vortech; Dearborn, MI).
Electrophysiological Testing
All compound action potentials (CAPs) were obtained using a PowerLab Data Acquisition System (ADInstruments; Colorado Springs, CO) interfaced with Scope™ 4 (ADInstruments; Colorado Springs, CO). CAPs were recorded prior to nerve transection (baseline) and after solution therapy. Electrophysiologic testing occurred at the rat’s normal body temperature, which was maintained with a warming blanket located underneath the rat. To accomplish this, a dual-terminal hook electrode was placed under the proximal and distal end of the exposed nerve, a minimum of 0.5 cm away from the coaptation site. In all animals, an electrical stimulus was delivered by the proximal electrode and was then recorded by the distal electrode. The parameters of stimulation were a delay of 5.0 mS, a stimulus of 50 mS at 40 kHz. The amount of volts applied to the nerve varied from 0.3 to 2.5 V. Once a compound action potential (CAP) was recorded, the pulse height was increased in increments until CAP amplitude seized to increase. This maximum CAP value was then recorded.
Behavioral Testing
Behavioral assessments were performed weekly until week 5; after which, rats were sacrificed at week 5 or 6.
Foot Fault Asymmetry Score
Animals were allowed to roam freely on a wire mesh grid measuring 45 cm × 30 cm, with square openings measuring 2.5 cm × 2.5 cm elevated 2 cm above a solid base. Trials were recorded for 50 total steps per hindlimb. Foot faults were scored when the hindlimb fell through the opening in the grid touching the base. If the hindlimb was retracted prior to touching the floor, a partial fault was scored. A composite fault score was calculated using a previously reported method [6, 28, 29]. The following equations were used:
-
1.
Composite Foot Fault score = (# Partial Faults × 1) + (# Full Faults × 2)
-
2.
% Foot Fault = (Composite Foot Fault score/total number of steps) × 100 %
-
3.
Foot Fault Asymmetry score = % Foot Fault (normal hindlimb) − % Foot Fault (surgical hindlimb)
Histology
Immunohistochemistry
Immunohistochemical staining was performed on rat sciatic nerves distal to the injury site on weeks 5 and 6 post-injury using commercial antibodies specifically directed against carbonic anhydrase II (CA-II) (Abcam; Cambridge, MA) and Choactase (Santa Cruz Biotechnology; Santa Cruz, CA). Carbonic anhydrase II staining and Choactase staining are positive in sensory and motor axons, respectfully [3, 7, 15, 17, 25]. Axial sections of the nerve were taken 3 to 5 mm distal to the coaptation site, which was roughly 15 mm from the rat sciatic notch. Formalin-fixed paraffin-embedded tissues were sectioned at 5 μm, placed on slides, and warmed overnight at 60 °C. Slides were deparaffinized and rehydrated with graded alcohols ending in Tris-buffered saline (TBS-T Wash Buffer, Lab Vision; Fremont, CA). For CA-II, heat-mediated target retrieval was performed in 1X Target Retrieval Buffer (pH 9.0, DAKO; Carpinteria, CA). Endogenous peroxidases and non-specific background were blocked by subsequent incubations in 3 % H2O2 (Fisher, Suwanee, GA) in TBS-T and serum-free protein block (RTU, DAKO). Primary antibody to CA-II was used 1:4000 for 1 h, followed by incubation in EnVision + HRP-labeled polymer (RTU, DAKO). For Choactase staining, no target retrieval was required. Endogenous peroxidases were blocked as before. Non-specific background, secondary, and tertiary labeling of target was accomplished by use of Vector’s ABC Elite Goat IgG kit (Vector Laboratories; Burlingame, CA). Primary antibody to Choactase was used at 1:35 for 1 h. Slides were rinsed with TBS-T between each reagent treatment, and all steps were carried out at room temperature unless otherwise noted. Visualization was achieved with DAB+ chromogen (DAKO). Slides were counterstained with Mayer’s hematoxylin, dehydrated through a series of alcohols and xylenes, and then coverslipped with Acrytol Mounting Media (Surgipath; Richmond, IL).
Light Microscopy
All stained slides were examined using an Olympus Vanox-T AH-2 light microscope (Olympus; Center Valley, PA) interfaced to Pixera Pro 600 HS digital camera (Pixera Corporation; Santa Clara, CA). Multiple digital photomicrographs were captured through a ×10 and ×5, CA-II and Choactase, respectively, objective using Viewfinder V3.0.1 (Pixera Corporation; Santa Clara CA). For each nerve processed, representative cross sections were photographed distal to the site of injury and distally from the sciatic nerve. To count the number of stained axons on each photomicrograph, ImageJ v1.45 software combined with the Wright Cell Imaging Facility plug-in package was used in a method that has been previously reported [1, 20]. Equivalent detection thresholds were used for counting axons in each photomicrograph. The total number of axons per cross section was determined by adding the axon totals from all representative photomicrographs or a given cross section; care was taken to avoid duplicate counting of axons.
Statistical Analyses
Statistical analyses were performed using Prism 5 (GraphPad Software; San Diego, CA). CAPs were compared by Mann–Whitney test. With regard to foot fault asymmetry scores, a Student’s t test was used for pairwise comparison. All p values were two-tailed, and significance was determined at p < 0.05.
Results
Electrophysiology Data
Electrophysiological testing revealed no significant difference in baseline CAPs and CAPs recorded in animals that received NT + PEG or NT alone (Fig. 1). Baseline CAPs were present in all, but one, animals (mean 4.86 ± 2.87 mV, minimum 0.0 mV, maximum 10.27 mV; n = 16). After nerve transection and immediate repair, CAPs could not be obtained in any of the animals repaired with nerve tubes alone (n = 7). CAPs were found in all PEG-treated animals (mean 4.47 ± 2.29 mV, min 1.50 mV, max 7.36 mV for PEG + NT (n = 9). Thus, the PEG-treated groups exhibited significantly improved CAPs compared to the NT alone group (p = 0.0002 for PEG + NT) (Fig. 1). No statistically significant difference between baseline and PEG + NT CAPs (p = 0.83) were observed.
Behavioral Data
Foot Fault Asymmetry Score
Foot fault asymmetry scores were significantly improved for NT + PEG compared to NT alone on postoperative days 7, 14, and 21 (p = 0.007, p = 0.001, and p = 0.006, respectively) (Fig. 2) However, no differences were noted between PEG + NT and NT alone on POD 28 and 35 (p = 0.35 and p = 0.89, respectively). Mean foot fault asymmetry scores with standard deviation are reported in Table 1. One rat from the experimental group died after anesthesia; therefore, behavioral or immunohistochemistry (IHC) data for that one rat does not exist.
Immunohistochemistry
Figure 3 shows representative nerve sections staining for axons using antibodies specific for CA-II roughly 5 to 6 weeks after injury. Figure 4 shows axon counts by CA-II in the nerve tube with PEG group as compared to the nerve tube without PEG group (1507 ± 221.5, n = 8; 888.4 ± 73.05, n = 7; p = 0.027). Figure 5 shows axon counts by Choactase in the nerve tube with PEG group as compared to the nerve tube without PEG group (1177 ± 105.9, n = 8; 762.3 ± 165.1, n = 7; p = 0.049).
Representative photomicrographs of sensory axons stained by carbonic anhydrase II protein, 5 to 6 weeks postoperatively, distal to the repair site comparing a nerve tube with PEG-treated nerve and b nerve tube without PEG-treated nerve. Photomicrographs of nerve tube with PEG-treated nerves were associated with a significantly (p < 0.05) increased number of sensory axons compared to nerve tube without PEG-treated nerve animals. Viable sensory axons are indicated by brown carbonic anhydrase II protein stain
Conduit-based application of PEG fusion confers improved axonal survival using antibiotic-specific carbonic anhydrase II (sensory axon) stain as compared to conduit without PEG. Nerve tube with PEG treatment group demonstrated a statistically significant increase in the number of axons as compared to the nerve tube without PEG treatment groups, between weeks 5 and 6 postoperatively using CA-II-specific antibody stains (1507 ± 222, n = 8 and 888 ± 73, n = 7 respectively; p = 0.027)
Conduit-based application of PEG fusion confers improved axonal survival using antibiotic-specific Choactase (motor axon) stain as compared to a conduit without PEG. Using Choactase-specific antibody stains of rat sciatic nerves, 5 to 6 weeks postoperatively, the nerve tube with PEG treatment group demonstrated a statistically significant increase in the number of axons as compared to the nerve tube without PEG treatment groups (1177 ± 106, n = 8 and 762 ± 165, n = 7 respectively; p = 0.049)
Discussion
Despite the recent advances in nerve repair that enhance the outgrowth of regenerating axons, it can still take months for regenerating axons to reach denervated target tissues after a proximal injury [8, 33]. The ability to immediately regain nerve function after injury to a segment of a peripheral nerve would revolutionize current nerve repair strategies. We have previously described a technique that allows early physiologic and behavioral recovery after nerve transection by fusing the cut ends of the axons back together with the hydrophilic polymer, polyethylene glycol (PEG) [4]. We then expanded that strategy and the current report describe how both physiologic and behavioral recovery can be immediately restored across a nerve gap using a fresh nerve autografts and this PEG fusion technique [29]. The key to this PEG-based axonal fusion is that it circumvents the delays associated with current nerve repair techniques which rely on axonal outgrowth and instead immediately fuses cut axons back together allowing more immediate recovery. In this study, we developed a delivery method using nerve conduits that both approximates the severed nerve edges and allows for easy application of the PEG fusion solutions.
Currently, microsutures are the primary repair strategy utilized clinically for peripheral nerve repair [8, 11]. Studies have suggested that microsutures evoke inflammatory reactions that may impair nerve recovery and even inhibit nerve conduction directly [5, 13, 27]. Recent advances with nerve fusion have continued to incorporate microsutures to demonstrate improved early and late electrophysiologic and behavioral recovery after traumatic neuropathy [4, 29]. However, the technique requires standard neurorrhaphy, followed by topical application of hydrophilic polymers to the cut axonal stumps. The polymer must diffuse across the epineurium, which if repaired too tightly, may impede delivery of the polymer to the potential axonal fusion sites. In an attempt to standardize and simplify delivery of the fusion milieu, we designed these experiments where only a few epineural stitches are applied, and then, a nerve tube is used to complete the repair and is used as a conduit for delivery of the fusion chemicals. We believe this is the first study directly comparing the results of nerve fusion via conduits at the repair site.
Nerve tubes were selected for use in comparison to microsuture because collagen conduits have demonstrated improved regeneration when utilized to repair nerve gaps [9, 12, 23, 26, 30]. For the purposes of this study, we used a collagen tube to prevent microsuture placement at the site of nerve repair and as a conduit for delivery of solutions. Our first notable finding is that PEG-mediated nerve fusion is possible with a collagen nerve tube and produces superior early electrophysiologic and 1-week behavioral outcomes (Figs. 1 and 2).
The behavioral data support the use of nerve tube with fusion as compared to controls. A statistically significant difference was noted up until 4 weeks postoperatively. At 4 weeks, there was not a significant difference, and by 5 weeks, there was overlap in their results. The control rats were repaired using the nerve tubes ensuring coaptation on the nerves. This allowed for axonal outgrowth from the proximal ends to the targeted muscle sites. This could account for the loss of significance in behavioral data starting at 4 weeks as previous studies have shown some degree of recovery in rats independent of treatment starting as early as 4 weeks after various types of injuries to the sciatic nerve [16, 21, 22].
Immunohistochemistry of the rat sciatic nerve axons at weeks 5 and 6 shows a statistically significant increase in the number of axons when the NT with PEG group is compared against the NT without PEG group (Figs. 4 and 5). Possible explanations are NT plus PEG allows for decreased neural death because of axonal fusion, our primary hypothesis, or it encourages accelerated growth by new axons. The presence of axons in the distal segment of the control rats could be accounted for by axonal outgrowth and correlates with the loss of behavioral difference at 4 and 5 weeks.
Limitations
Although our behavioral results are exciting until 4 weeks postoperatively, the long-term PEG fusion results have resulted in no long-term improvement. The IHC shows a statistically significant difference between the experimental group and the control group, but not one that correlates with behavioral differences. This is most likely attributed to axonal outgrowth in such a short nerve with eventual reinnervation in our control animals. Although a larger animal model might confirm the effectiveness of PEG fusion, other possibilities such as PEG- or MB-related toxicity and/or scarring may play a role in the moderate long-term outcome improvements.
Another confounding factor is that fusion supersedes the reinnervation specificity seen with axonal outgrowth. Adjacent axons simply fuse to one another base on spatial proximity. We expect that graft rotation with non-specific alignment and fusion of axons may have detrimental effects on the animal’s eventual outcome and, if severe, may actually interfere with recovery. A final limitation to this technique is that if Wallerian degeneration has progressed sufficiently, axonal fusion will not be possible. Although we have been able to delay fusion up to 24 h after injury in our sciatic injury model (data not shown), the maximum delay between injury and PEG fusion that will produce a superior outcome has yet to be determined.
Summary
Hydrophilic polymer-based nerve fusion shows continued promise as a technique to enhance nerve injury outcomes in animal models. The enhancements to PEG nerve fusion described in this study not only improved early electrophysiological outcomes compared to standard nerve repair but also showed behavioral differences up until 4 weeks post-injury. Conduit-based PEG application warrants further evaluation due to its effectiveness and ease of application and because of its potential in proximal human injuries to recover function in cases where current nerve repair strategies yield consistently poor outcomes.
References
Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophoton Int. 2004;11(7):36–43.
Archibald SJ, Shefner J, Krarup C, Madison RD. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J Neurosci Off J Soc Neurosci. 1995;15(5 Pt 2):4109–23.
Badia J, Pascual-Font A, Vivó M, Udina E, Navarro X. Topographical distribution of motor fascicles in the sciatic-tibial nerve of the rat. Muscle Nerve. 2010;42(2):192–201.
Bittner GD, Keating CP, Kane JR, Britt JM, Spaeth CS, Fan JD, et al. Rapid, effective, and long-lasting behavioral recovery produced by microsutures, methylene blue, and polyethylene glycol after completely cutting rat sciatic nerves. J Neurosci Res. 2012;90(5):967–80.
Boedts D. A comparative experimental study on nerve repair. Arch Otorhinolaryngol. 1987;244(1):1–6.
Britt JM, Kane JR, Spaeth CS, Zuzek A, Robinson GL, Gbanaglo MY, et al. Polyethylene glycol rapidly restores axonal integrity and improves the rate of motor behavior recovery after sciatic nerve crush injury. J Neurophysiol. 2010;104(2):695–703.
Cammer W, Tansey FA. Immunocytochemical localization of carbonic anhydrase in myelinated fibers in peripheral nerves of rat and mouse. J Histochem Cytochem. 1987;35(8):865–70.
Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119(9):1951–65.
Chamberlain LJ, Yannas IV, Hsu HP, Strichartz G, Spector M. Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp Neurol. 1998;154(2):315–29.
Deriemer SA, Elliott EJ, Macagno ER, Muller KJ. Morphological evidence that regenerating axons can fuse with severed axon segments. Brain Res. 1983;272(1):157–61.
Diao E, Vannuyen T. Techniques for primary nerve repair. Hand Clin. 2000;16(1):53–66. viii.
Inada Y, Morimoto S, Takakura Y, Nakamura T. Regeneration of peripheral nerve gaps with a polyglycolic acid-collagen tube. Neurosurgery. 2004;55(3):640–6. discussion 646-648.
Inaloz SS, Ak HE, Vayla V, Akin M, Aslan A, Sari I, et al. Comparison of microsuturing to the use of tissue adhesives in anastomosing sciatic nerve cuts in rats. Neurosurg Rev. 1997;20(4):250–8.
Kantor TG. Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy. 1986;6(3):93–103.
Kawasaki Y, Yoshimura K, Harii K, Park S. Identification of myelinated motor and sensory axons in a regenerating mixed nerve. J Hand Surg. 2000;25(1):104–11.
Koka R, Hadlock TA. Quantification of functional recovery following rat sciatic nerve transection. Exp Neurol. 2001;168(1):192–5.
Lago N, Navarro X. Correlation between target reinnervation and distribution of motor axons in the injured rat sciatic nerve. J Neurotrauma. 2006;23(2):227–40.
Li ST, Archibald SJ, Krarup C, Madison RD. Peripheral nerve repair with collagen conduits. Clin Mater. 1992;9(3-4):195–200.
Lore AB, Hubbell JA, Bobb Jr DS, Ballinger ML, Loftin KL, Smith JW, et al. Rapid induction of functional and morphological continuity between severed ends of mammalian or earthworm myelinated axons. J Neurosci Off J Soc Neurosci. 1999;19(7):2442–54.
Marina N, Bull ND, Martin KR. A semiautomated targeted sampling method to assess optic nerve axonal loss in a rat model of glaucoma. Nat Protoc. 2010;5(10):1642–51.
Mazzer PY, Barbieri CH, Mazzer N, Fazan VP. Morphologic and morphometric evaluation of experimental acute crush injuries of the sciatic nerve of rats. J Neurosci Methods. 2008;173(2):249–58.
Monte-Raso VV, Barbieri CH, Mazzer N, Yamasita AC, Barbieri G. Is the Sciatic Functional Index always reliable and reproducible? J Neurosci Methods. 2008;170(2):255–61.
Nakamura T, Inada Y, Fukuda S, Yoshitani M, Nakada A, Itoi S, et al. Experimental study on the regeneration of peripheral nerve gaps through a polyglycolic acid-collagen (PGA-collagen) tube. Brain Res. 2004;1027(1-2):18–29.
Neumann B, Nguyen KC, Hall DH, Ben-Yakar A, Hilliard MA. Axonal regeneration proceeds through specific axonal fusion in transected C. elegans neurons. Dev Dyn. 2011;240(6):1365–72.
Riley DA, Sanger JR, Matloub HS, Yousif NJ, Bain JL, Moore GH. Identifying motor and sensory myelinated axons in rabbit peripheral nerves by histochemical staining for carbonic anhydrase and cholinesterase activities. Brain Res. 1988;453(1):79–88.
Rosen JM, Padilla JA, Nguyen KD, Siedman J, Pham HN. Artificial nerve graft using glycolide trimethylene carbonate as a nerve conduit filled with collagen compared to sutured autograft in a rat model. J Rehabil Res Dev. 1992;29(2):1–12.
Sames M, Blahos Jr J, Rokyta R, Benes Jr V. Comparison of microsurgical suture with fibrin glue connection of the sciatic nerve in rabbits. Physiol Res. 1997;46(4):303–6.
Sedy J, Urdzikova L, Jendelova P, Sykova E. Methods for behavioral testing of spinal cord injured rats. Neurosci Biobehav Rev. 2008;32(3):550–80.
Sexton KW, Pollins AC, Cardwell NL, Del Corral GA, Bittner GD, Shack RB, et al. Hydrophilic polymers enhance early functional outcomes after nerve autografting. J Surg Res. 2012;177(2):392–400.
Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery. 2010;30(7):574–88.
Stanec S, Tonkovic I, Stanec Z, Tonkovic D, Dzepina I. Treatment of upper limb nerve war injuries associated with vascular trauma. Injury. 1997;28(7):463–8.
Waitayawinyu T, Parisi DM, Miller B, Luria S, Morton HJ, Chin SH, et al. A comparison of polyglycolic acid versus type 1 collagen bioabsorbable nerve conduits in a rat model: an alternative to autografting. J Hand Surg [Am]. 2007;32(10):1521–9.
Wolf SW, Hotchkiss RN, Pederson WC, Kozin SH. Green’s operative hand surgery. Philadelphia: Churchill Livingstone; 2011.
Source of Funding
The basis of this work was supported by the Vanderbilt CTSA grant UL1 RR024975-01 from NCRR/NIH. This work was also supported by the Department of Defense OR120216 “Development of Class II Medical Device for Clinical Translation of a Novel PEG Fusion Method for Immediate Physiological Recovery after Peripheral Nerve Injury.”
Conflict of Interest
Kevin W. Sexton and Wesley P. Thayer have a provisional patent related to the technology discussed in this manuscript.
Charles L. Rodriguez-Feo declares that he has no conflict of interest.
Richard B. Boyer declares that he has no conflict of interest.
Gabriel A. Del Corral declares that he has no conflict of interest.
David C. Riley declares that he has no conflict of interest.
Alonda C. Pollins declares that she has no conflict of interest.
Nancy L. Cardwell declares that she has no conflict of interest.
R. Bruce Shack declares that he has no conflict of interest.
Lillian B. Nanney declares that she has no conflict of interest.
Statement of Human and Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Statement of Informed Consent
Not applicable
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sexton, K.W., Rodriguez-Feo, C.L., Boyer, R.B. et al. Axonal fusion via conduit-based delivery of hydrophilic polymers. HAND 10, 688–694 (2015). https://doi.org/10.1007/s11552-015-9780-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11552-015-9780-9