Abstract
Purpose
A new method for acetabular cartilage segmentation in both computed tomography (CT) arthrography and magnetic resonance imaging (MRI) datasets with leg tension is developed and tested.
Methods
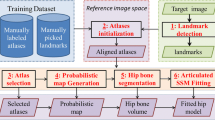
The new segmentation method is based on the combination of shape and intensity information. Shape information is acquired according to the predictable nonlinear relationship between the U-shaped acetabulum region and acetabular cartilage. Intensity information is obtained from the acetabular cartilage region automatically to complete the segmentation procedures. This method is evaluated using 54 CT arthrography datasets with two different radiation doses and 20 MRI datasets. Additionally, the performance of this method in identifying acetabular cartilage is compared with four other acetabular cartilage segmentation methods.
Results
This method performed better than the comparison methods. Indeed, this method maintained good accuracy level for 74 datasets independent of the cartilage modality and with minimum user interaction in the bone segmentation procedures. In addition, this method was efficient in noisy conditions and in detection of the damaged cartilages with zero thickness, which confirmed its potential clinical usefulness.
Conclusions
Our new method proposes acetabular cartilage segmentation in three different datasets based on the combination of the shape and intensity information. This method executes well in situations where there are clear boundaries between the acetabular and femoral cartilages. However, the acetabular cartilage and pelvic bone information should be obtained from one dataset such as CT arthrography or MRI datasets with leg traction.












Similar content being viewed by others
References
Lane NE (2007) Osteoarthritis of the hip. N Engl J Med 357(14):1413–1421
Nishii T, Sugano N, Sato Y, Tanaka H, Miki H, Yoshikawa H (2004) Three-dimensional distribution of acetabular cartilage thickness in patients with hip dysplasia: a fully automated computational analysis of mr imaging. Osteoarthr Cartil 12(8):650–657
Tamura S, Nishii T, Shiomi T, Yamazaki Y, Murase K, Yoshikawa H, Sugano N (2012) Three-dimensional patterns of early acetabular cartilage damage in hip dysplasia; a high-resolutional CT arthrography study. Osteoarthr Cartil 20(7):646–652
Williams TG, Holmes AP, Waterton JC, Maciewicz RA, Hutchinson CE, Moots RJ, Nash AFP, Taylor CJ (2010) Anatomically corresponded regional analysis of cartilage in asymptomatic and osteoarthritic knees by statistical shape modelling of the bone. IEEE Trans Med Imaging 29(8):1541–1559
Cheng Y, Wang S, Yamazaki T, Zhaob J, Nakajima Y, Tamura S (2007) Hip cartilage thickness measurement accuracy improvement. Comput Med Imaging Graph 31(8):643–655
Siversson C, Akhondi-Asl A, Bixby S, Kim YJ, Warfield SK (2014) Three-dimensional hip cartilage quality assessment of morphology and dGEMRIC by planar maps and automated segmentation. Osteoarthr Cartil 22(10):1511–1515
Tabrizi PR, Zoroofi RA, Yokota F, Tamura S, Nishii T, Sato Y (2015) Acetabular cartilage segmentation based on bone-normalized probabilistic atlas from contrast-enhanced CT images. Int J Comput Assist Radiol Surg 10(4):433–446
Cheong J, Suter D, Cicuttini F (2005) Development of semi-automatic segmentation methods for measuring tibial cartilage volume. In: Proceedings of DICTA, pp 307–314
Fripp J, Crozier S, Warfield SK, Ourselin S (2010) Automatic segmentation and quantitative analysis of the articular cartilages from magnetic resonance images of the knee. IEEE Trans Med Imaging 29(1):55–64
Glocker B, Komodakis N, Paragios N, Glaser C, Tziritas G, Navab N (2007) Primal/dual linear programming and statistical atlases for cartilage segmentation. Proc MICCAI 10:536–543
Folkesson J, Dam E, Olsen O, Pettersen P, Christiansen C (2007) Segmenting articular cartilage automatically using a voxel classification approach. IEEE Trans Med Imaging 26(1):106–115
Lee S, Park S, Shim H, Yun I, Lee S (2011) Optimization of local shape and appearance probabilities for segmentation of knee cartilage in 3-D MR images. Comput Vis Image Underst 115(12):1710–1720
Zhang K, Lu W, Marziliano P (2013) Automatic knee cartilage segmentation from multi-contrast MR images using support vector machine classification with spatial dependencies. Magn Reson Imaging 31(10):1731–1743
Baniasadipour A, Zoroofi RA, Sato Y, Nishii T, Tanaka H (2011) Automated knowledge-based segmentation and analysis of the hip bones and cartilages using multi-slice CT data. Imaging Sci 59(5):253–266
Khanmohammadi M, Zoroofi RA, Nishii T, Tanaka H, Sato Y (2009) A hybrid technique for thickness-map visualization of the hip cartilages in MRI. IEICE Trans Inf Syst E92-D(11):2253–2263
Du X, Velut J, Bolbos R, Beuf O, Odet C, Benoit-Cattin H (2008) 3-D knee cartilage segmentation using a smoothing b-spline active surface. In: Proceedings of ICIP, pp 2924–2927
Ali-Shah SA, Yahya K, Mubashar G, Bais A (2010) Quantification and visualization of MRI cartilage of the knee: a simplified approach. In: Proceedings of ICET, pp 175–180
Rantalainen M, Bylesjo M, Cloarec O, Nicholson JK, Holmes E, Trygg J (2007) Kernel-based orthogonal projections to latent structures (K-OPLS). J Chemom 21(7–9):376–385
Bylesjo M, Rantalainen M, Nicholson JK, Holmes E, Trygg J (2008) K-OPLS package: kernel-based orthogonal projections to latent structures for prediction and interpretation in feature space. BMC Bioinform 9(106):1–7
Fonville JM, Bylesjö M, Coen M, Nicholson JK, Holmes E, Lindon JC, Rantalainen M (2011) Non-linear modeling of 1h NMR metabonomic data using kernel-based orthogonal projections to latent structures optimized by simulated annealing. Anal Chim Acta 705(1–2):72–80
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26(3):299–302
Yokota F, Okada T, Takao M, Sugano N, Tada Y, Sato Y (2009) Automated segmentation of the femur and pelvis from 3D CT data of diseased hip using hierarchical statistical shape model of joint structure. Proc MICCAI 12:811–818
National Library of Medicine Insight Segmentation and Registration Toolkit. http://www.itk.org
Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ (1999) Nonrigid registration using free-form deformations: application to breast MR images. Trans Med Imaging 18(8):712–721
Carr JC, Beatson RK, Cherrie JB, Mitchell TJ, Fright WR, McCallum BC, Evans TR (2001) Reconstruction and representation of 3D objects with radial basis functions. In: Proceedings of SIGGRAPH, pp 67–76
Zhang N, Zhang J, Shi R (2008) An improved Chan-Vese model for medical image segmentation. Proc Comput Sci Softw Eng 1:864–867
Heimann T, van Ginneken B, Styner MA, Arzhaeva Y, Aurich V, Bauer C, Beck A, Becker C, Beichel R, Bekes G, Bello F, Binnig G, Bischof H, Bornik A, Cashman PM, Chi Y, Cordova A, Dawant BM, Fidrich M, Furst JD, Furukawa D, Grenacher L, Hornegger J, Kainmüller D, Kitney RI, Kobatake H, Lamecker H, Lange T, Lee J, Lennon B, Li R, Li S, Meinzer HP, Nemeth G, Raicu DS, Rau AM, van Rikxoort EM, Rousson M, Rusko L, Saddi KA, Schmidt G, Seghers D, Shimizu A, Slagmolen P, Sorantin E, Soza G, Susomboon R, Waite JM, Wimmer A, Wolf I (2009) Comparison and evaluation of methods for liver segmentation from CT datasets. IEEE Trans Med Imaging 28(8):1251–1265
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
For this type of study, formal consent is not required because this study is a retrospective study.
Informed consent
Written informed consent was not required for this study because this study is a retrospective study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tabrizi, P.R., Zoroofi, R.A., Yokota, F. et al. Shape-based acetabular cartilage segmentation: application to CT and MRI datasets. Int J CARS 11, 1247–1265 (2016). https://doi.org/10.1007/s11548-015-1313-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-015-1313-z