Abstract
Purpose
Unfortunately, the current re-excision rates for breast conserving surgeries due to positive margins average 20–40 %. The high re-excision rates arise from difficulty in localizing tumor boundaries intraoperatively and lack of real-time information on the presence of residual disease. The work presented here introduces the use of supine magnetic resonance (MR) images, digitization technology, and biomechanical models to investigate the capability of using an image guidance system to localize tumors intraoperatively.
Methods
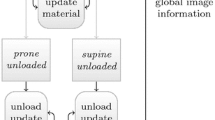
Preoperative supine MR images were used to create patient-specific biomechanical models of the breast tissue, chest wall, and tumor. In a mock intraoperative setup, a laser range scanner was used to digitize the breast surface and tracked ultrasound was used to digitize the chest wall and tumor. Rigid registration combined with a novel nonrigid registration routine was used to align the preoperative and intraoperative patient breast and tumor. The registration framework is driven by breast surface data (laser range scan of visible surface), ultrasound chest wall surface, and MR-visible fiducials. Tumor localizations by tracked ultrasound were only used to evaluate the fidelity of aligning preoperative MR tumor contours to physical patient space. The use of tracked ultrasound to digitize subsurface features to constrain our nonrigid registration approach and to assess the fidelity of our framework makes this work unique. Two patient subjects were analyzed as a preliminary investigation toward the realization of this supine image-guided approach.
Results
An initial rigid registration was performed using adhesive MR-visible fiducial markers for two patients scheduled for a lumpectomy. For patient 1, the rigid registration resulted in a root-mean-square fiducial registration error (FRE) of 7.5 mm and the difference between the intraoperative tumor centroid as visualized with tracked ultrasound imaging and the registered preoperative MR counterpart was 6.5 mm. Nonrigid correction resulted in a decrease in FRE to 2.9 mm and tumor centroid difference to 5.5 mm. For patient 2, rigid registration resulted in a FRE of 8.8 mm and a 3D tumor centroid difference of 12.5 mm. Following nonrigid correction for patient 2, the FRE was reduced to 7.4 mm and the 3D tumor centroid difference was reduced to 5.3 mm.
Conclusion
Using our prototype image-guided surgery platform, we were able to align intraoperative data with preoperative patient-specific models with clinically relevant accuracy; i.e., tumor centroid localizations of approximately 5.3–5.5 mm.









Similar content being viewed by others
References
Adkisson CD, Bagaria SP, Parker AS, Bray JM, Gibson T, Thomas CS, Heckman MG, McLaughlin SA (2012) Which eligible breast conservation patients choose mastectomy in the setting of newly diagnosed breast cancer? Ann Surg Oncol 19(4):1129–1136
Alderliesten T, Loo C, Paape A, Muller S, Rutgers E, Peeters MJV, Gilhuijs K (2010) On the feasibility of mri-guided navigation to demarcate breast cancer for breast-conserving surgery. Med Phys 37(6):2617–2626
Allweis TM, Kaufman Z, Lelcuk S, Pappo I, Karni T, Schneebaum S, Spector R, Schindel A, Hershko D, Zilberman M, Sayfan J, Berlin Y, Hadary A, Olsha O, Paran H, Gutman M, Carmon M (2008) A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery. Am J Surg 196(4):483–489
Besl PJ, McKay ND (1992) Method for registration of 3-d shapes. In: Robotics-DL tentative. International Society for Optics and Photonics, pp 586–606
Braun M, Pölcher M, Schrading S, Zivanovic O, Kowalski T, Flucke U, Leutner C, Park-Simon TW, Rudlowski C, Kuhn W, Kuhl CK (2008) Influence of preoperative mri on the surgical management of patients with operable breast cancer. Breast Cancer Res Treat 111(1):179–187
Buxant F, Scuotto F, Hottat N, Noël JC, Simon P (2007) Does preoperative magnetic resonance imaging modify breast cancer surgery? Acta chirurgica Belgica 107(3):288
Carter T, Tanner C, Beechey-Newman N, Barratt D, Hawkes D (2008) Mr navigated breast surgery: method and initial clinical experience. In: Medical image computing and computer-assisted intervention-MICCAI 2008. Springer, pp 356–363
Conley RH, Meszoely IM, Pheiffer TS, Weis JA, Yankeelov TE, Miga MI (2014) Image to physical space registration of supine breast mri for image guided breast surgery. In: SPIE Medical Imaging. International Society for Optics and Photonics, p 90362N
Davis KM, Hsu CH, Bouton ME, Wilhelmson KL, Komenaka IK (2011) Intraoperative ultrasound can decrease the re-excision lumpectomy rate in patients with palpable breast cancers. Am Surg 77(6):720–725
Ebrahimi M, Siegler P, Modhafar A, Holloway CM, Plewes DB, Martel AL (2014) Using surface markers for mri guided breast conserving surgery: a feasibility survey. Phys Med Biol 59(7):1589
Fancher TT, Palesty JA, Thomas R, Healy T, Fancher JM, Ng C, Dudrick SJ (2009) A woman’s influence to choose mastectomy as treatment for breast cancer. J Surg Res 153(1):128–131
Fisher B, Bauer M, Margolese R, Poisson R, Pilch Y, Redmond C, Fisher E, Wolmark N, Deutsch M, Montague E, Saffer E, Wickerham L, Lerner H, Glass A, Shibata H, Deckers P, Ketcham A, Oishi R, Russel I (1985) Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 312(11):665–673
Glossop ND (2009) Advantages of optical compared with electromagnetic tracking. J Bone Jt Surg 91(Supplement 1):23–28
Han L, Hipwell J, Eiben B, Barratt D, Modat M, Ourselin S, Hawkes D (2014) A nonlinear biomechanical model based registration method for aligning prone and supine mr breast images. Med Imaging IEEE Trans 33(3):682–694
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA: Cancer J Clinicians 61(2):69–90
Landercasper J, Whitacre E, Degnim AC, Al-Hamadani M (2014) Reasons for re-excision after lumpectomy for breast cancer: insight from the american society of breast surgeons masterysm database. Ann Surg Oncol 21(10):3185–3191
Lorensen WE, Cline HE (1987) Marching cubes: a high resolution 3d surface construction algorithm. In: ACM siggraph computer graphics, vol 21. ACM, pp 163–169
Mortensen EN, Barrett WA (1995) Intelligent scissors for image composition. In: Proceedings of the 22nd annual conference on computer graphics and interactive techniques, pp 191–198
Muratore DM, Galloway RL Jr (2001) Beam calibration without a phantom for creating a 3-d freehand ultrasound system. Ultrasound Med Biol 27(11):1557–1566
Pallone MJ, Poplack SP, Avutu HBR, Paulsen KD, Barth Jr RJ (2014) Supine breast mri and 3d optical scanning: a novel approach to improve tumor localization for breast conserving surgery. Ann Surg Oncol 21(7):2203–2208
Pheiffer TS, Simpson AL, Lennon B, Thompson RC, Miga MI (2012) Design and evaluation of an optically-tracked single-ccd laser range scanner. Med Phys 39(2):636–642
Pheiffer TS, Thompson RC, Rucker DC, Simpson AL, Miga MI (2014) Model-based correction of tissue compression for tracked ultrasound in soft tissue image-guided surgery. Ultrasound Med Biol 40(4):788–803
Pleijhuis RG, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM (2009) Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol 16(10):2717–2730
Rucker D, Wu Y, Clements L, Ondrake J, Pheiffer T, Simpson A, Jarnagin W, Miga M (2014) A mechanics-based nonrigid registration method for liver surgery using sparse intraoperative data. Med Imaging IEEE Trans 33(1):147–158
Siegler P, Holloway C, Causer P, Thevathasan G, Plewes DB (2011) Supine breast mri. J Magn Reson Imaging 34(5):1212–1217
Sonka M, Fitzpatrick JM (2000) Handbook of medical imaging(volume 2, medical image processing and analysis). SPIE- The International Society for Optical Engineering
Sullivan JM Jr, Charron G, Paulsen KD (1997) A three-dimensional mesh generator for arbitrary multiple material domains. Finite Elem Anal Des 25(3):219–241
Tafra L, Fine R, Whitworth P, Berry M, Woods J, Ekbom G, Gass J, Beitsch P, Dodge D, Han L, Potruch T, Francescatti D, Oetting L, Smith JS, Snider H, Kleban D, Chagpar A, Akbari S (2006) Prospective randomized study comparing cryo-assisted and needle-wire localization of ultrasound-visible breast tumors. Am J Surg 192(4):462–470
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3d active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31(3):1116–1128
Acknowledgments
We would like to acknowledge the support of the Vanderbilt Initiative in Surgery and Engineering Pilot Award Program for support of this work. This work was partially funded by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences and in part by a National Science Foundation Graduate Research Fellowship award awarded to RHC. We thank the National Institutes of Health for funding through NCI U01CA174706. Finally, we thank the Kleberg Foundation for the generous support of our Imaging Institute.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conley, R.H., Meszoely, I.M., Weis, J.A. et al. Realization of a biomechanical model-assisted image guidance system for breast cancer surgery using supine MRI. Int J CARS 10, 1985–1996 (2015). https://doi.org/10.1007/s11548-015-1235-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-015-1235-9