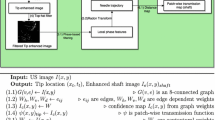
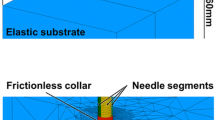
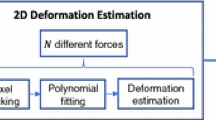
Abstract
Purpose
Tissue compression during ultrasound imaging leads to error in the location and geometry of subsurface targets during soft tissue interventions. We present a novel compression correction method, which models a generic block of tissue and its subsurface tissue displacements resulting from application of a probe to the tissue surface. The advantages of the new method are that it can be realized independent of preoperative imaging data and is capable of near-video framerate compression compensation for real-time guidance.
Methods
The block model is calibrated to the tip of any tracked ultrasound probe. Intraoperative digitization of the tissue surface is used to measure the depth of compression and provide boundary conditions to the biomechanical model of the tissue. The tissue displacement field solution of the model is inverted to nonrigidly transform the ultrasound images to an estimation of the tissue geometry prior to compression. This method was compared to a previously developed method using a patient-specific model and within the context of simulation, phantom, and clinical data.
Results
Experimental results with gel phantoms demonstrated that the proposed generic method reduced the mock tumor margin modified Hausdorff distance (MHD) from \(5.0\,\pm \,1.6\) to \(2.1\,\pm \,0.7\,\hbox {mm}\) and reduced mock tumor centroid alignment error from \(7.6\,\pm \,2.6\) to \(2.6\,\pm \,1.1\,\hbox {mm}\). The method was applied to a clinical case and reduced the in vivo tumor margin MHD error from \(5.4\,\pm \,0.1\) to \(2.9\,\pm \,0.1\,\hbox {mm}\), and the centroid alignment error from \(7.2\,\pm \,0.2\) to \(3.8\,\pm \,0.4\,\hbox {mm}\).
Conclusions
The correction method was found to effectively improve alignment of ultrasound and tomographic images and was more efficient compared to a previously proposed correction.











Similar content being viewed by others
References
Treece GM, Gee AH, Prager RW (2005) RF and amplitude-based probe pressure correction for 3D ultrasound. Ultrasound Med Biol 31(4):493–503. doi:10.1016/j.ultrasmedbio.2004.12.018
Xiao G, Brady JM, Noble JA, Burcher M, English R (2002) Nonrigid registration of 3-D free-hand ultrasound images of the breast. IEEE Trans Med Imaging 21(4):405–412. doi:10.1109/TMI.2002.1000264
Burcher MR, Lianghao H, Noble JA (2001) Deformation correction in ultrasound images using contact force measurements. In: IEEE workshop on mathematical methods in biomedical image analysis (MMBIA), 2001, pp 63–70. doi:10.1109/mmbia.2001.991700
Sun S-Y (2010) Deformation correction in ultrasound imaging in an elastography framework. Thesis S.M.—Massachusetts Institute of Technology Dept. of Electrical Engineering and Computer Science 2010, Massachusetts Institute of Technology, Cambridge
Pheiffer TS, Thompson RC, Rucker DC, Simpson AL, Miga MI (2014) Model-based correction of tissue compression for tracked ultrasound in soft tissue image-guided surgery. Ultrasound Med Biol. doi:10.1016/j.ultrasmedbio.2013.11.003
Rucker DC, Wu Y, Clements LW, Ondrake JE, Pheiffer TS, Simpson AL, Jarnagin WR, Miga MI (2013) A mechanics-based nonrigid registration method for liver surgery using sparse intraoperative data. IEEE Trans Med Imaging 33(1):147–158. doi:10.1109/tmi.2013.2283016
Lange T, Papenberg N, Heldmann S, Modersitzki J, Fischer B, Lamecker H, Schlag PM (2009) 3D ultrasound-CT registration of the liver using combined landmark-intensity information. Int J Comput Assist Radiol Surg 4(1):79–88. doi:10.1007/s11548-008-0270-1
Chen I, Ong RE, Simpson AL, Sun K, Thompson RC, Miga MI (2013) Integrating retraction modeling into an atlas-based framework for brain shift prediction. IEEE Trans Biomed Eng 60(12):3494–3504. doi:10.1109/Tbme.2013.2272658
Coffey AM, Miga MI, Chen I, Thompson RC (2013) Toward a preoperative planning tool for brain tumor resection therapies. Int J Comput Assist Radiol Surg 8(1):87–97. doi:10.1007/s11548-012-0693-6
Bro-Nielsen M (1998) Finite element modeling in surgery simulation. Proc IEEE 86(3):490–503. doi:10.1109/5.662874
Sullivan JM, Charron G, Paulsen KD (1997) A three-dimensional mesh generator for arbitrary multiple material domains. Finite Elem Anal Des 25(3–4):219–241. doi:10.1016/S0168-874x(96)00027-3
Arun KS, Huang TS, Blostein SD (1987) Least-squares fitting of 2 3-D point sets. IEEE Trans Pattern Anal Mach Intell 9(5):699–700
Besl PJ, Mckay ND (1992) A method for registration of 3-D shapes. IEEE Trans Pattern Anal Mach Intell 14(2):239–256
Muratore DM, Galloway RL (2001) Beam calibration without a phantom for creating a 3-D freehand ultrasound system. Ultrasound Med Biol 27(11):1557–1566
Pheiffer TS, Simpson AL, Lennon B, Thompson RC, Miga MI (2012) Design and evaluation of an optically-tracked single-CCD laser range scanner. Med Phys 39(2):636–642. doi:10.1118/1.3675397
Dubuisson MP, Jain AK (1994) A modified Hausdorff distance for object matching. In: Proceedings of the 12th IAPR international conference on pattern recognition, vol 1—conference A: computer vision and image processing, vol 561, pp 566–568, 9–13 Oct 1994. doi:10.1109/icpr.1994.576361
Dumpuri P, Thompson RC, Dawant BM, Cao A, Miga MI (2007) An atlas-based method to compensate for brain shift: preliminary results. Med Image Anal 11(2):128–145. doi:10.1016/j.media.2006.11.002
Miller KE, Ondrake JE, Pheiffer TS, Simpson AL, Miga MI (2012) Utilizing ultrasound as a surface digitization tool in image guided liver surgery. In: David RH III, Kenneth HW (eds) SPIE, p 83163D
Kumar AN, Miga MI, Pheiffer TS, Chambless LB, Thompson RC, Dawant BM (2015) Persistent and automatic intraoperative 3D digitization of surfaces under dynamic magnifications of an operating microscope. Med Image Anal 19(1):30–45. doi:10.1016/j.media.2014.07.004
Lange T, Eulenstein S, Hunerbein M, Lamecker H, Schlag PM (2004) Augmenting intraoperative 3D ultrasound with preoperative models for navigation in liver surgery. In: Medical image computing and computer-assisted intervention-Miccai 2004, Pt 2, proceedings, 3217:534–541
Acknowledgments
This work was supported in part by the National Institutes of Health award R01 NS049251 of the National Institute for Neurological Disorders and Stroke, and by the National Institutes of Health award R01 CA162477 from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pheiffer, T.S., Miga, M.I. Toward a generic real-time compression correction framework for tracked ultrasound. Int J CARS 10, 1777–1792 (2015). https://doi.org/10.1007/s11548-015-1210-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-015-1210-5