Abstract
Objectives
To determine key MDCT features for characterizing pancreatic neuroendocrine tumours (PNET) from their mimics, which manifest as enhancing pancreatic mass with normal serum CA19-9 level.
Methods
This retrospective study had institutional review board approval and informed consent was waived. Preoperative multiphase MDCT of 74 patients with enhancing pancreatic masses and normal serum CA19-9 levels were included. Surgical pathologies were PNET (n = 42), microcystic serous cystadenomas (m-SCN, n = 12) and solid pseudopapillary epithelial neoplasms (SPEN, n = 20). Two radiologists independently evaluated CT images with a checklist of findings. Frequencies of findings with each disease entity were compared. Diagnostic accuracy was assessed using the key MDCT features alone and in combination. Inter-observer agreement was evaluated.
Results
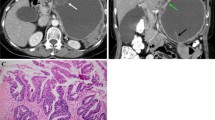
The most common findings for PNET were mosaic morphological pattern (33/42, 78.6%) and enhancement peak in pancreatic arterial phase (PAP, 32/42, 76.2%), for m-SCN were honeycomb pattern (9/12, 75.0%) and enhancement peak in PAP (10/12, 83.3%) and for SPEN were melting icecream pattern (16/20, 80.0%) and hypo-enhancement in all phases (18/20, 90.0). Using a combination of morphological patterns and enhancement features, PNET was identified with 88% sensitivity and 81% specificity, m-SCN was identified with 83% sensitivity and 94% specificity, and SPEN was identified with 90% sensitivity and 91% specificity. Inter-observer agreement concerning CT findings was good to excellent (κ = 0.68 to 0.81, all p < 0.01).
Conclusions
Morphological features and enhancement patterns on MDCT are key features for characterizing enhancing pancreatic mass with normal serum CA19-9. PNET could be differentiated from its mimics with high accuracy.




Similar content being viewed by others
References
Marinelli T, Filippone A, Tavano F, Fontana A, Pellegrini F et al (2014) A tumour score with multidetector spiral CT for venous infiltration in pancreatic cancer: influence on borderline resectable. Radiol Med 119:334–342
Hu S, Huang W, Lin X, Wang Y, Chen KM et al (2014) Solid pseudopapillary tumour of the pancreas: distinct patterns of computed tomography manifestation for male versus female patients. Radiol Med 119:83–89
Foti G, Boninsegna L, Falconi M, Mucelli RP (2013) Preoperative assessment of nonfunctioning pancreatic endocrine tumours: role of MDCT and MRI. Radiol Med 118:1082–1101
Jang SK, Kim JH, Joo I, Jeon JH, Shin KS et al (2015) Differential diagnosis of pancreatic cancer from other solid tumours arising from the periampullary area on MDCT. Eur Radiol 25:2880–2888
Vitali F, Hansen T, Kiesslich R, Heinrich S, Kumar A et al (2014) Frequency and characterization of benign lesions in patients undergoing surgery for the suspicion of solid pancreatic neoplasm. Pancreas 43:1329–1333
Hyodo R, Suzuki K, Ogawa H, Komada T, Naganawa S (2015) Pancreatic neuroendocrine tumours containing areas of iso- or hypoattenuation in dynamic contrast-enhanced computed tomography: spectrum of imaging findings and pathological grading. Eur J Radiol 84:2103–2109
Tatsumoto S, Kodama Y, Sakurai Y, Shinohara T, Katanuma A et al (2013) Pancreatic neuroendocrine neoplasm: correlation between computed tomography enhancement patterns and prognostic factors of surgical and endoscopic ultrasound-guided fine-needle aspiration biopsy specimens. Abdom Imaging 38:358–366
Humphrey PE, Alessandrino F, Bellizzi AM, Mortele KJ (2015) Non-hyperfunctioning pancreatic endocrine tumours: multimodality imaging features with histopathological correlation. Abdom Imaging 40:2398–2410
Baek JH, Lee JM, Kim SH, Kim SJ, Lee JY et al (2010) Small (<or=3 cm) solid pseudopapillary tumours of the pancreas at multiphasic multidetector CT. Radiology 257:97–106
D’Onofrio M, De Robertis R, Capelli P, Tinazzi Martini P, Crosara S et al (2015) Uncommon presentations of common pancreatic neoplasms: a pictorial essay. Abdom Imaging 40:1629–1644
Sahani DV, Kambadakone A, Macari M, Takahashi N, Chari S et al (2013) Diagnosis and management of cystic pancreatic lesions. AJR Am J Roentgenol 200:343–354
Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR et al (2005) Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics 25:1471–1484
Kainuma O, Yamamoto H, Cho A, Arimitsu H, Yanagibashi H et al (2015) Solid variant type of serous cystadenocarcinoma of the pancreas: a case report and review of the literature. Pancreatology 15:197–199
Park HS, Kim SY, Hong SM, Park SH, Lee SS et al (2016) Hypervascular solid-appearing serous cystic neoplasms of the pancreas: Differential diagnosis with neuroendocrine tumours. Eur Radiol 26:1348–1358
Choi JY, Kim MJ, Lee JY, Lim JS, Chung JJ et al (2009) Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol 193:136–142
Hayashi K, Fujimitsu R, Ida M, Sakamoto K, Higashihara H et al (2012) CT differentiation of solid serous cystadenoma vs endocrine tumour of the pancreas. Eur J Radiol 81:e203–e208
Malleo G, Bassi C, Rossini R, Manfredi R, Butturini G et al (2012) Growth pattern of serous cystic neoplasms of the pancreas: observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut 61:746–751
Bassi C, Salvia R, Molinari E, Biasutti C, Falconi M et al (2003) Management of 100 consecutive cases of pancreatic serous cystadenoma: wait for symptoms and see at imaging or vice versa? World J Surg 27:319–323
Papavramidis T, Papavramidis S (2005) Solid pseudopapillary tumours of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 200:965–972
Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR et al (1996) Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology 199:707–711
Buetow PC, Parrino TV, Buck JL, Pantongrag-Brown L, Ros PR et al (1995) Islet cell tumours of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR Am J Roentgenol 165:1175–1179
Sun HY, Kim SH, Kim MA, Lee JY, Han JK et al (2010) CT imaging spectrum of pancreatic serous tumours: based on new pathologic classification. Eur J Radiol 75:e45–e55
Park MJ, Lee JH, Kim JK, Kim YC, Park MS et al (2014) Multidetector CT imaging features of solid pseudopapillary tumours of the pancreas in male patients: distinctive imaging features with female patients. Br J Radiol 87:20130513
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was funded by the National Natural Science Foundation of China (General Program, No.81371608) and the Health Industry Special Scientific Research Project (No. 201402019).
Author-identifying information
Hua-dan Xue has received funding from the National Natural Science Foundation of China (General Program, No.81371608) and author Zheng-yu Jin has received funding from Health Industry Special Scientific Research Project (No. 201402019). The authors (L. Zhu, H. Xue, W. Liu,. Wang, X. Sui, Q. Wang, D. Zhang, P. Li and Z. Jin) declare no relationships with any companies, whose products or services may be related to the subject matter of this article.
Ethical approval
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This retrospective study was approved by the institutional review board of Peking Union Medical College Hospital, and written informed consent was waived.
Rights and permissions
About this article
Cite this article
Zhu, L., Xue, Hd., Liu, W. et al. Enhancing pancreatic mass with normal serum CA19-9: key MDCT features to characterize pancreatic neuroendocrine tumours from its mimics. Radiol med 122, 337–344 (2017). https://doi.org/10.1007/s11547-017-0734-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0734-x