Abstract
We propose a mathematical model to investigate the transmission dynamics of Rift Valley fever (RVF) virus among ruminants. Our findings indicate that in endemic areas RVF virus maintains at a very low level among ruminants after outbreaks and subsequent outbreaks may occur when new susceptible ruminants are recruited into endemic areas or abundant numbers of mosquitoes emerge when herd immunity decreases. Many factors have been shown to have impacts on the severity of RVF outbreaks; a higher probability of death due to RVF among ruminants, a higher mosquito:ruminant ratio, or a shorter lifespan of animals can amplify the magnitude of the outbreaks; vaccination helps to reduce the magnitude of RVF outbreaks and the loss of animals efficiently, and the maximum vaccination effort (a high vaccination rate and a larger number of vaccinated animals) is recommended before the commencement of an outbreak but can be reduced later during the enzootic.
Similar content being viewed by others
1 Introduction
The Rift Valley fever virus (RVFV), a member of the Phlebovirus genus in the Bunyaviridae family, significantly affects a wide range of animal species including domestic ruminants by causing massive abortions in pregnant animals and death in young animals, especially in the case of sheep. More than 30 mosquito species in the genera Aedes, Anopheles, Culex, Eretmapoites, and Mansonia, and other arthropods including sand flies are responsible for transmission among domestic ruminants (Fontenille et al. 1998). However, Aedes and Culex mosquitoes are considered the primary vectors (Abdo-Salem et al. 2011). Due to animal trade and numerous species of mosquitoes that can transmit RVFV, there is a growing concern that it will emerge further in non-endemic areas, including the United States (Kasari et al. 2008).
RVFV was first isolated in Kenya in the early 1930s (Daubney et al. 1931). Since then it has been encountered in enzootic or epizootic forms across Africa, from Senegal to Madagascar and from Egypt to South Africa (Abdo-Salem et al. 2011). RVFV has two distinct transmission cycles: low-level endemic and epidemic (Hollidge et al. 2010). During the endemic cycle, when there is non-excessive rainfall, it is believed that in East Africa RVFV is maintained through vertical transovarial transmission in Aedes mosquito eggs (Linthicum et al. 1985). However, this is not the case for Culex mosquitoes as they oviposit egg rafts that soon hatch, and vertical transovarial transmission is not currently present in the Middle East and West Africa. The transition from the low-level enzootic cycle to the epidemic cycle is often associated with heavy and prolonged rainfall and flooding events that allow the emergence of large numbers of primary vectors, Aedes mosquitoes, and the subsequent population increases of Culex mosquitoes that serve as excellent secondary vectors (Hollidge et al. 2010). Climate variability is a determinant of mosquito-borne RVFV transmission (Gage et al. 2008), including events such as the occurrence of the El Niño/Southern Oscillation (ENSO) phenomenon (Linthicum et al. 1999). In addition, animal movements/migration/smuggling, agricultural development, dam building, irrigation, or other human activity may also facilitate the virus dissemination (Balkhy and Memish 2003).
Currently, two types of vaccines are available for animals: inactivated whole-virus and live-attenuated Smithburn vaccines (Davies 2006). Inactivated vaccines can be applied to ruminants of all ages without causing abortions but they are expensive and repeated doses are required. On the other hand, live-attenuated vaccines are cheap and effective. They confer a lifelong immunity with a single dose. However, they may lead to fetal abnormalities and abortions in pregnant ruminants and there is the safety concern of reversion to virulence (Ikegami and Makino 2009).
Many modeling tools have been used to investigate the risk of RVF resurgence in the endemic areas and the risk of disease introduction in disease-free areas including climatic indices, spatial techniques, multivariable statistical analysis, and dynamical transmission models (Métras et al. 2011; Linthicum et al. 1999; Anyamba et al. 2009; Favier et al. 2006; Bicout and Sabatier 2004; Gaff et al. 2007; Mpeshe et al. 2011; Xue et al. 2012; Niu et al. 2012; Barker et al. 2013; Chitnis et al. 2013; Gao et al. 2013; Manore and Beechler 2013; Xue et al. 2013). In this modeling approach to investigate the emergence of RVF outbreaks and epizootic and enzootic cycles of RVFV, we address some aspects that have not been considered in previous mathematical modeling studies. Here, we propose a mathematical model that takes into account the possibility of abortions in diseased ruminants due to RVFV. We investigate the transmission dynamics of RVFV and show that certain parameters are relevant to the start of an outbreak, the prevalence of RVFV and the epidemic size of an outbreak. We study whether seasonality in mosquito abundance, unusually excessive rainfall events, recruitment of susceptible ruminants, and low mosquito activity can prompt subsequent RVF outbreaks. Furthermore, to delineate optimal vaccination strategies, we incorporate vaccination and study how the delay in its implementation affects the transmission dynamics of RVFV.
2 Methods
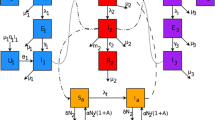
Mathematical modeling is employed to investigate the transmission dynamics of RVFV. Our model is based on a framework for vector-borne diseases with ruminants as hosts and mosquitoes as vectors. The intrinsic incubation period of RVFV in ruminants is approximately 1–6 days and the extrinsic incubation period of RVFV in mosquitoes is approximately 4–8 days (Turell and Kay 1998; Gaff et al. 2007). As the addition of an exposed class is akin to introducing a slight time delay in the system and the incubation period is relatively short when compared to host or vector lifespan in our case, for simplicity as is similar to several malaria models we do not include exposed classes of ruminants and mosquitoes (Gupta et al. 1994; Childs and Boots 2010). Ruminants are divided into three categories: susceptible \((S)\), infectious \((I)\), and recovered (or immune to RVFV) \((R)\). Female mosquitoes are divided into two categories: uninfected \((U)\) and infectious \((V)\), under the assumption that mosquitoes remain infectious for life and infection does no harm to them. A flow diagram is depicted in Fig. 1.
2.1 Model Assumptions
To construct the model, we make the following assumptions:
-
(1)
The ruminant population is described by the logistic growth equation with \(b\) as a birth rate (where female:male ratio is already taken into account), \(\mu \) as a natural death rate (including with and without slaughter), and \(q\) as a parameter reflecting density dependence of the total population of ruminants on the birth rate or reflecting limited resources or human demands in the area. This assumption allows fluctuations of the ruminant population due to resources and RVFV. Since infection with RVFV causes high rates of abortion in ruminants, it is assumed that births from infectious ruminants are reduced by a proportion \((1-r)\).
-
(2)
We assume that each female mosquito bites at a constant rate \((a)\) distributed uniformly among ruminants within the area. Hence, transmission is frequency-dependent with respect to the ruminant population and the rate at which a particular ruminant is bitten by a particular mosquito is \(a/N\), where \(N\) is the total number of ruminants (\(N=S+I+R\)) (Keeling and Rohani 2007). Infection in a ruminant by an infectious bite of a mosquito is successful with a probability of \(p_\mathrm{{r}}\). A mosquito is infected during its blood meal from an infectious ruminant with a probability of \(p_\mathrm{{m}}\).
-
(3)
Because infection with RVFV also causes high mortality in ruminants, we assume that ruminants die due to RVFV infection at a rate of \(d\) and we also assume that this rate includes slaughtering sick animals.
-
(4)
Infection with RVFV induces life-long virus neutralizing immunity in humans and animals (Barnard 1979; Swanepoel et al. 1986; Paweska et al. 2005). We assume that ruminants recover with life-long immunity by natural clearance at a rate of \(\gamma \).
-
(5)
For simplicity, it is assumed that the mosquito population is homogeneous and abundant, and that its population size \((M)\) depends on two quantities, the mosquito:ruminant ratio \((k)\) and the number of ruminants in a population \((N)\), and that \(M=kN\). First, let us assume initially that the total number of mosquitoes is a constant so that \(M = k_0N_0\), where \(k_0\) is the constant mosquito:ruminant ratio and \(N_0\) is the maximum size of the ruminant population \((N_0=\underset{0\le t<\infty }{\max } N(t))\). Mosquito abundance can be thought of as driven by the amount of precipitation. Hence, second, we may take into account seasonality in mosquito abundance in our work by assuming that the mosquito:ruminant ratio fluctuates over time as a sinusoidal function such that the mosquito:ruminant ratio is highest at the middle of the wet season and lowest at the middle of the dry season as follows
$$\begin{aligned} k = k_1(1-k_2\cos {2\pi t}), \end{aligned}$$with \(k_{\max }={\mathop {\max }_{0\le t\le 2\pi }} k(t)\), \(k_{\min }={\mathop {\min }_{0\le t\le 2\pi }} k(t)\), \(k_1=(k_{\max }+k_{\min })/2\), \(k_2 = (k_{\max }/k_{\min }-1)/(k_{\max }/k_{\min }+1)\), and \(M=kN\) (Altizer et al. 2006; Childs and Boots 2010). Note that this second assumption is slightly different from the previous assumption in that not only \(k\) varies over time but also \(N\) does as well. Hence, when an outbreak occurs and the number of ruminants decreases following the spread of RVFV, the total number of mosquitoes is consequently reduced. Also note that although this work does not focus on vector control, some strategies such as applying larvicide or destroying mosquito breeding sites may relate to the mosquito:ruminant ratio and could be considered.
-
(6)
The recruitment rate of mosquitoes is assumed as \(\eta M\), where \(\eta \) is a natural birth/death rate of mosquitoes.
-
(7)
By taking vertical transmission of RVFV in mosquitoes into account, we assume that a proportion \(p\) of their offspring emerge infected with RVFV and consequently \((1-p)\) emerge as not infected but susceptible. In areas in which vertical transmission is not present, \(p\) can be set to zero.
2.2 Baseline Model
The assumptions lead to the following system of equations:
Model parameter values can be found in Table 1.
2.3 Model with Vaccination
To reduce the loss of animals and consequent economic burden, vaccination is one of the important means currently used for controlling the spread of RVFV. In an attempt to understand how vaccination alters the dynamics of RVFV among ruminants and mosquitoes, we incorporate vaccination into the baseline model. We assume that only susceptible ruminants are vaccinated at a rate of \(\phi \) (\(1/\phi \) is assumed as a period required for vaccinating a whole susceptible population and it is assumed that this period is short in accordance with the small number of ruminant population in our study. If the total number of ruminant population is bigger, this quantity may become very large.). For simplicity, we assume that after successful vaccination, ruminants are completely immune to the virus and move to the recovery compartment with life-long immunity. Hence, when vaccination is taken into account, a term \(\phi S\) is subtracted from the RHS of \(\dot{S}\) and added to the RHS of \(\dot{R}\) in the baseline model.
2.4 Basic Reproduction Number
Without the RVFV transmission, we have
Obviously, the number of ruminants does not change if \(N(0)=0\) and \(N(0)=N_0=(b-\mu )/q\). For \(0 < N(0) < (b-\mu )/q\), it increases, and for \(N(0) > (b-\mu )/q\), it decreases.
Without vertical transmission in mosquitoes \((p=0)\), there are two biologically relevant equilibria at \(E_0 = (N_0,0,0,M,0)\) and \(E^* = (S^*,I^*,R^*,U^*,V^*)\). Note that the trivial equilibrium of the form \((0, 0, 0, M, 0)\) is always unstable. When vertical transmission in mosquitoes takes place \((0 < p \le 1 )\), there is only a disease-present equilibrium \((S^*,I^*,R^*,U^*,V^*)\). We do not consider the trivial equilibrium of the form \((0, 0, 0, (1-p)M, pM)\) that is always unstable.
To calculate the basic reproductive number of (1) when vertical transmission is rare, we use a method of the next generation matrices developed in Diekmann et al. (2010) and van den Driessche and Watmough (2002). We introduce two vectors, \(\mathcal {F}\) and \(\mathcal {V}\), to represent the new and transported infections into the two infected states \((I,V)\) as follows:
The Jacobian matrices of \(\mathcal {F}\) and \(\mathcal {V}\) at the disease-free equilibrium \((E_0)\) are denoted by \(F\) and \(V\) (respectively) and described as follows:
Consequently, \(R_0\) can be obtained from the maximum eigenvalue of \(FV^{-1}\) at the disease-free equilibrium \((E_0)\):
Hence, the basic reproductive number of (1) is
where \(N_0=(b-\mu )/q\). Note that we use a square of the maximum eigenvalue as \(R_0\). When vaccination is present, the basic reproductive number is
2.5 Optimal Vaccination
Now the vaccination rate is assumed to vary with time \((\phi =\phi (t))\). Our goal is to try to find an optimal vaccination strategy that minimizes the number of infectious ruminants. We follow the steps of solving the optimal control problem in (Lenhart and Workman 2007).
We consider an optimal vaccination to minimize the following objective functional:
Note that \(\phi ^2\) is introduced to prevent a bang-bang control to occur and also reflect the minimization of the vaccination control itself. Based on this objective functional, we wish to find an optimal vaccination rate that minimizes the number of infectious ruminants in the limited time interval [0,T]. The objective functional is subjected to the system (1) incorporated by vaccination that can be described in terms of vector notations as follows:
where
and \(0\le \phi \le \phi ^{\max }\). Introducing five adjoint variables, we have the Hamiltonian as
The adjoint and transversality conditions are
Because the control is bounded, the optimality condition is
where \(\phi ^{\max }\) is the upper bound of vaccination rate. To solve this problem, the forward-backward sweep method is used.
3 Results
3.1 Baseline Model
When there is no vertical transmission in mosquitoes, two possibilities can occur, with either RVFV dying out or persisting. RVFV goes extinct under the condition \(R_0 = R_\mathrm{{r}}R_\mathrm{{m}} < 1\), where \(R_\mathrm{{r}}=ap_\mathrm{{r}}/(\mu +d+\gamma )\) is the number of infective contacts for transmission from ruminant to mosquito and \(R_\mathrm{{m}}=ap_\mathrm{{m}}k/\eta \) is the number of infective contacts for transmission from mosquito to ruminant. From this condition, persistence of RVFV depends on transmission rates of RVFV from mosquitoes to ruminants and vice versa (\(ap_\mathrm{{r}}\) and \(ap_\mathrm{{m}}\)), birth rate of ruminants through \(k_0\), natural death rates of ruminants and mosquitoes, disease-related death rate in ruminants, and recovery rate. Figure 2a and b shows that without vertical transmission in mosquitoes, RVFV dies out when \(R_0<1\) and is endemic when \(R_0>1\). When vertical transmission in mosquitoes occurs, RVFV persists even though \(R_0<1\) (Fig. 2c). Note that parameter values used throughout this result section (unless stated) can be found in Table 1 and \((S(0),I(0),R(0),U(0),V(0)) = (49800,200,0,19800,200)\) and \((S(0),I(0),R(0),U(0),V(0))=(49900,95,5,74900,100)\) are initial conditions for \(R_0=0.85\) and \(R_0=17\), respectively, used in numerical simulations throughout this study.
Persistence of RVFV. Numerical solutions of infectious ruminants \(I(t)\) and mosquitoes \(V(t)\): (a, b) without vertical transmission in mosquitoes \((p=0)\) when \(R_0=0.85\) \((a=146, p_m=0.2, k_0=0.4)\) and \(17\), respectively, (c) with vertical transmission in mosquitoes \((p=0.05)\) when \(R_0=0.85\). RVFV dies out when there is no vertical transmission and \(R_0<1\) and it is endemic when there is no vertical transmission and \(R_0>1\). Although \(R_0<1\), RVFV is endemic when there is vertical transmission
When \(R_0>1\), the numbers of ruminants and mosquitoes in each disease status are shown in Fig. 3 when there is vertical transmission. Our results suggest that an outbreak takes place with approximately 10-week duration (this result is close to 12–16 weeks long in semi arid zones (Davies 2010)) and it does not subsequently reappear without a change in underlying conditions as the virus maintains itself at a very low level afterward. Based on our results, the number of susceptible ruminants drastically decreases during the outbreak as susceptible ruminants are infected with RVFV rapidly and infectious ruminants that survive become immune to the virus eventually. Correspondingly, the number of susceptible mosquitoes decreases during the outbreak but later increases as ruminants acquire immune protection against the virus and hence do not transmit RVFV. The number of infectious mosquitoes increases dramatically during the outbreak (with a small delay as compared with the infectious ruminants) and later decreases and remains at a low level after the outbreak. Figure 4a and b shows that the probability of RVF-related death in ruminants and the mosquito:ruminant ratio play a crucial role in the epidemic size of RVFV (or the maximum number of infectious ruminants during an outbreak) in such a way that when these quantities are higher (especially the latter one), it may lead to a higher number of incidences of RVF during the outbreak and endemic period. By further investigating the long-term behaviors of RVFV among ruminants when two important parameters (the birth rate and probability of RVF-related death) relating to the ruminant population vary, we find that the numbers of susceptible and recovered ruminants are large when there are enough births of ruminants and the RVFV-related death probability is small (Fig. 4c, e). Figure 4d implies that after an outbreak, the prevalence of RVFV (or the number of infectious ruminants at the disease-present equilibrium divided by the total population of ruminants) is high when the birth rate is high and the related death probability is intermediate.
The epidemic size and the long-term behaviors of populations The epidemic size (or the maximum number of infectious ruminants during an outbreak) when the birth rate and probability of death due to RVF vary (a) and the mosquito:ruminant ratio and the mosquito lifespan vary (b). The number of ruminants of each disease status when the birth rate and probability of death due to RVF vary and \(t\) tends to \(\infty \) (or the number of ruminants at the equilibrium): (c) susceptible, (d) infectious, and (e) recovered (Color figure online)
3.2 Recruitment of Ruminants/Seasonality/Abnormal Weather
To investigate subsequent outbreaks, here we define a subsequent outbreak as another major outbreak with substantial numbers of infectious ruminants compared to an endemic level and other small outbreaks nearby. We assume that temporarily there are a very large number of mosquitoes to explore whether such can cause subsequent outbreaks. Motivated by the possibility that ruminant owners may maintain the size of their herds in the area by importing new susceptible ruminants to the herds after the outbreak(s), we introduce new susceptible ruminants into the ruminant population at the beginning of every year or every 5th year in simulation studies of our model. These periods can be adjusted to account for the banning of imported animals by government after an outbreak occurs. This event can be captured by a Dirac delta function \(\sum _{n=1}^{\infty }(N^0-N(t))\delta (t-n\tau ),\) where \(\tau \) is a fixed period of introduction, \(n=1,2,3,\ldots \), and \(\delta \) is a Dirac delta function such that \(\delta (t-n\tau )=1\) when \(t=n\tau \) and \(\delta (t-n\tau )=0\) elsewhere. This pulse of animal recruitment can be added into the equation for \(\dot{S}\) to represent an introduction of susceptible animals at a particular point.
Figure 5a shows that when there is an introduction of susceptible ruminants in the area, there is the probability of a few subsequent outbreaks with declining RVF incidences occurring after the first major outbreak before the prevalence of RVFV becomes very low. Figure 5b shows the total number of ruminants resulting from the recruitment of susceptible ruminants at the beginning of every year versus every 5th year. It suggests that although recruitment of ruminants at the beginning of every 5th year may lead to a greater loss of ruminants in subsequent outbreaks when compared with annual introductions, the number of animals being introduced is lower. Note that this conclusion is based on counting the number of newly introduced ruminants at the recruitment points and does not compare the total economic gain from remaining ruminants and the cost of animal introduction. By including seasonality of mosquito abundance as a sinusoidal function via the mosquito:ruminant ratio, the number of mosquitoes changes over time during dry and wet seasons (Fig. 5c). Because we assume that the mosquito:ruminant ratio is the highest at the middle of each wet season and the lowest at the middle of each dry season, we find that after a major outbreak, the prevalence of RVFV remains at a very low level among ruminants and is time-periodic with a peak near the middle of every wet season (Fig. 5d).
Seasonality and abnormality of weather. The number of infectious ruminants (a) and the total number of ruminants (b) when there is recruitment of susceptible ruminants every year (red and solid trace) and every 5th year (blue and dashed trace). When seasonality of mosquito abundance is taken into account, (c) shows the number of mosquitoes per ruminants over time (\(k_{\min }=0.2, k_{\max }=6\)) and (d) shows the corresponding number of infectious ruminants to seasonal forcing. Assume that there is seasonality in mosquito abundance and abnormality of weather and \(k(t) = k_1(1-k_2\cos {2\pi t}), \;0\le t\le 5.3,5.7\le t\le 10\) and \(k(t) = k^*, \;5.3\le t\le 5.7\) with \(k^*=10\). (e) Gives the number of infectious ruminants when there is recruitment of ruminants at every year (blue trace), every 5th year (green trace), and no recruitment of ruminants (red trace) and (f) shows the number of infectious ruminants if there is recruitment of ruminants at every 5th year and when the probability of death due to RVFV \((m)\) varies at 0.3 (green trace), 0.6 (magenta trace), and 0.9 (blue trace) (Color figure online)
Since the transition from a low-level in enzootic cycle of RVFV to an epidemic cycle may relate to the abnormality of weather due to heavy and prolonged rainfall and flooding events, we investigate how such an event alters the spread of RVFV by assuming that seasonality of mosquito abundance is present as a sinusoidal function. At the 5th year the mosquito:ruminant ratio is amplified lasting for approximately 5 months and straightening over the middle of the year. For the rest of the year, the mosquito:ruminant ratio corresponds to the sinusoidal function. In this scenario, we find that without recruitment of ruminants, subsequent outbreaks may not occur among ruminants after the first outbreak (Fig. 5e). When there is recruitment of ruminants every year or every 5th year, subsequent outbreaks may take place (also Fig. 5e). One of the important parameters in the model that relates to the high prevalence of RVFV is the probability of death due to the disease (\(m\)). Figure 5f shows that by varying this parameter, the higher probability of RVF-related death in ruminants may cause a higher number of infectious ruminants in subsequent outbreak(s).
We further assume that the mosquito abundance is a step function that reflects a strong dependence on suitable weather conditions so that there are only 4 rainy months in the middle of the year with high numbers of mosquitoes and 8 dry months with very low numbers of mosquitoes. As shown in Fig. 6a, RVFV maintains at a very low level with small outbreaks and peaks at the wet seasons. However, when we assume extreme cases of weather conditions in which mosquito activity nearly disappears due to droughts for longer periods such as two, three, or five years, it may result in subsequent outbreaks (Fig. 6b). When the RVF-related death probability varies, Fig. 6c shows that the higher the virulence the lower the number of incidences in the second outbreak. This result is in contrast to the case in which there is recruitment of susceptible ruminants into the ruminant population. When ruminants have shorter lifespan, it may lead to the higher number of incidences in subsequent outbreaks (Fig. 6d).
High and low mosquito activity. When there is no recruitment of animals, a very low level of mosquito activity (\(k=0.05\)) for certain periods of time may cause subsequent outbreaks: (a) the prevalence of RVF is very low within a year of low level of mosquito activity; (b) subsequent outbreaks occur when the period of low level of mosquito activity is extended (black trace = 2 years, blue trace = 3 years, red trace = 5 years); (c) the higher probability of death due to RVFV, the lower number of RVF incidences during subsequent outbreak (black, red, blue traces for \(m\) = 0.3, 0.6, 0.9, respectively); (d) the shorter lifespan of animal herds may cause more serious subsequent outbreaks (black, red, blue traces for \(\mu \) = 1/3,1/5.7,1/7, respectively) (Color figure online)
3.3 Vaccination
When vaccination is implemented, the basic reproductive number becomes
This formula suggests that a higher rate of vaccination helps to reduce the likelihood that an outbreak takes place and maintains in the populations. Figure 7a shows that the higher rate of vaccination helps to decrease the epidemic size of RVFV among ruminants, and it also helps to reduce the loss of ruminants due to the virus (Fig. 7b). When delay in the administration of vaccine is approximately 2 months, the epidemic size is approximately the same size as when there is no vaccination (Fig. 7c). However, the total number of ruminants is higher when vaccination is present compared to no vaccination (Fig. 7d). As shown in Fig. 7c and d, with one month delay the epidemic size decreases and the loss of ruminant population is reduced. Furthermore, without a delay in vaccination the outbreak incidences are drastically reduced, and the total number of ruminants remains very large. Because RVFV remains very low in the populations after an outbreak in our predictions, it may be useful to consider a time varying vaccination rate to gain insight of possible ways to control the spread of RVFV efficiently.
Vaccination. High vaccination rates help to reduce the maximum number of infectious ruminants (a) and the loss of ruminants (b). The delay in vaccination before an outbreak may lead to (c) the higher number of infectious ruminants, and (d) the lower number of of ruminants in total (red and solid trace = without vaccination, blue and dotted trace = with delay in 2 months, green and dashed trace = with delay in 1 month, and black and dashed-dotted trace = without delay in vaccination). When the vaccination rate varies with time to minimize the number of infectious ruminants, (e) shows the optimal vaccination rate, while (f) and (g) show the corresponding numbers of infectious ruminants and entire ruminants, respectively (red and solid trace = with \(\phi ^{max} = 365/10\), blue and dotted trace = with \(\phi ^{max} =365/15\), and green and dashed trace = with \(\phi ^{max} =365/20\)) (Color figure online)
To further investigate this strategy, we use an optimal control technique (Lenhart and Workman 2007), assuming that the vaccination rate depends on time. The objective goal is to minimize the number of infectious ruminants. Hence, the objective function is
Figure 7e–g shows the optimal vaccination rate, the number of infectious ruminants, and the total number of ruminants when the maximum rate of vaccination varies. Our results suggest that it may be efficient to attempt the maximum vaccination rate at the beginning before an outbreak starts and that such a maximal attempt at vaccination may help to reduce the epidemic size of the RVF outbreak. Also, we observe that the higher rate of vaccination, the shorter time needed to administer vaccine at a maximal rate. As time passes, once RVFV is endemic at a very low level, it may be possible to reduce the vaccination rate. However, based on our ranges of parameters, RVFV cannot be eliminated by the intervention but remains at a very low level.
4 Discussion
We have developed a simple modeling framework to capture transmission of RVFV among ruminants via mosquitoes. This mathematical framework was used to investigate the transmission dynamics of RVFV, in particular, how certain parameters influence the prevalence of RVFV and epidemic size of an outbreak and how seasonality, unusually excessive rainfall events and recruitment of susceptible ruminants affect the occurrence of RVF subsequent outbreaks. Moreover, we studied how vaccination and a delay in its implementation affect the transmission dynamics of RVFV.
4.1 Baseline Model
Our model predictions are that: (\(i\)) without considerations such as peculiar weather events or recruitment of ruminants when there is no vertical transmission, RVFV is endemic if \(R_0>1\) and more likely remains at a very low level after an outbreak, and it dies out if \(R_0<1\); (\(ii\)) the probability of RVF-related death and mosquito:ruminant ratio play an important role in the epidemic size; and (\(iii\)) the birth rate and RVF-related death probability of ruminants may be important predictors to determine the total size of ruminant population and the prevalence of RVFV after an outbreak.
Prediction (\(i\)) corresponds to several empirical studies in many endemic areas that show 1–3 % of domesticated animals being infected with RVFV in certain areas of Africa during non-epizootic periods (Davies et al. 1992; Rostal et al. 2010), while this percentage is as low as 0.1 % in Yemen (Abdo-Salem et al. 2011). Death of animals due to RVFV infection varies among ages and types of animals. The mortality rate of lambs is up to 90 % while it is nearly 10–30 % in adult sheep and 20 % in cattle (Bird et al. 2009; Kortekaas et al. 2010). Based on our predictions that a higher probability of RVF-related death may lead to a larger number of incidences during epizootic and enzootic periods, these may suggest that areas with young animals and susceptible breeds of sheep or cattle are more likely to exhibit a serious outbreak of RVF. Because of births of animals acting as a source of (susceptible) ruminants in our model, without enough births the ruminant population may die out after an outbreak. Note that abortions of animals due to RVFV may become important as a means of reducing the number of births in areas with a large number of pregnant animals. Also, prediction (\(ii\)) suggests that if the probability of RVF-related death is very high, the ruminant population may go extinct. On the other hand, if it is too low, fewer individuals are likely to be infected and a higher number of recovered and susceptible individuals is to be expected. Hence, these two quantities may act as important predictors to determine the total size of ruminant population and the prevalence of RVFV after the outbreak.
4.2 Recruitment of Ruminants/Seasonality/Abnormal Weather
In areas in which RVFV keeps circulating, RVF outbreaks are periodic and occur approximately every 10–15 or 3–7 years in some endemic regions (Andriamandimby et al. 2010; Murithi et al. 2011). Although we varied certain parameters, our model does not predict subsequent outbreaks but instead it suggests very low prevalence of RVFV after the outbreak (as our solutions tend to the disease-present equilibrium rather quickly due to the high number of infected animals becoming immune to the virus) which corresponds to empirical findings (Davies et al. 1992; Rostal et al. 2010; Abdo-Salem et al. 2011). Hence, we considered certain factors that may have impacts on subsequent outbreaks. When seasonality of mosquito abundance is incorporated in our model, the mosquito:ruminant ratio varies periodically according to wet and dry seasons. Our predictions suggest that: (\(iv\)) recruitment (or movement) of susceptible ruminants to the area may cause subsequent outbreaks with declining incidences and although recruiting ruminants in every 5th year for example may lead to a greater loss of ruminants in subsequent outbreaks than recruiting every year, the number of animal introductions in total is lower; (\(v\)) with seasonality in mosquito abundance, the prevalence of RVFV remains at a very low level among ruminants after the outbreak and changes over time during dry and wet seasons in a pattern that is similar to seasonal forcing in mosquitoes; (\(vi\)) when there is seasonality of mosquito activity and abnormal weather, subsequent outbreaks may not occur without substantial numbers of susceptible ruminants; and (\(vii\)) a very low level of mosquito activity for certain periods of time may cause subsequent outbreaks although new sources of ruminants are not recruited.
In this study, recruitment of ruminants represents another source of susceptible individuals to the population apart from natural births. Alternatively, recruitment can be regarded as animal movement to the area. When an outbreak occurs, many ruminants die due to RVFV so that recruitment of ruminants may involve massive numbers of animals relative to the live births in the area (Thiongane et al. 1997). Under the presence of RVFV in mosquitoes, predictions (\(iv\))–(\(vi\)) suggest that recruitment of susceptible ruminants may lead to subsequent outbreaks. This conclusion links to some studies suggesting that cattle of owners who purchase animals to replace their herds following outbreaks were significantly more antibody-positive than others and outbreaks are likely to occur when heavy rainfall and flooding coincides with the time required for herd immunity to fall to permissable levels (Chevalier et al. 2011; Murithi et al. 2011). RVF virus activity occurs annually and is associated with seasonal rains during non-epidemic periods (Davies et al. 1992) and this corresponds to our prediction \((v)\). When there is seasonality in mosquito abundance and abnormal weather, outbreaks may not take place without a new source of susceptible ruminants to decrease the herd immunity. However, as other findings in our work suggest that outbreaks are more serious when the mosquito:ruminant ratio is high and animals have shorter lifespan, further detailed investigations are needed.
It has been suggested that the population turnover of ruminants, as herders favor recruitment of animals and intensive animal reproduction to recover dramatic loss by death and abortion from an outbreak, may increase the risk of an epidemic (Thiongane et al. 1997). Our modeling results show that a higher probability of RVF-related death in ruminants may cause a larger number of infectious ruminants during the outbreaks. The more animals that die from the outbreaks, the larger the number recruited as replacements and consequently the lower the immunity to the virus in the herds and the higher the risk of outbreaks. Also, whenever the virus is introduced to the native area (under suitable conditions) or new susceptible ruminants are introduced to the endemic areas, serious outbreaks are more likely to take place. This conclusion is supported by previous findings suggesting that the introduction of viraemia animals into an area with high numbers of susceptible animals and potential vectors can create an epizootic (Gad et al. 1986). Based on prediction (\(vii\)), when there is no recruitment of ruminants, the very low level of mosquito activity at certain periods of time due to abnormal weather may cause subsequent outbreaks. Note that the very low level of mosquito activity may be associated with water scarcity, unsuitable conditions for mosquitoes, efficient mosquito control programme, or animal movement from the area. During the very low level of mosquito activity, natural births of animals may play an important role in introducing new susceptible ruminants to the area. The longer the low number of mosquitoes that transmit RVFV, the larger the number of susceptible ruminants. As there are very few new individuals infected and immune to the virus during a sustained period of very low level of mosquito activity, an event introducing numbers of mosquitoes to the areas when there are enough number of susceptible ruminants may lead to subsequent outbreaks. Furthermore, when ruminants are not recruited to the area, our predictions suggest that RVF-related deaths from the previous outbreak and the longevity of ruminants may determine the number of recovered ruminants for the herd immunity and the number of susceptible ruminants left for subsequent outbreaks. Although prediction (\(vii\)) is supported by real events in many areas in which epizootics took place after a prolonged dry season was interrupted by heavy and widespread rainfall (Anyamba et al. 2002; Weaver and Reisen 2010), further detailed investigations that consider relations between the prolonged dry periods (or the periods with very low mosquito activity in our work), animal movement in the area, the year’s stock of mosquito eggs and other factors are needed.
4.3 Vaccination
When vaccination is incorporated into the model, our predictions are as follows: (\(viii\)) a higher rate of vaccination may help to reduce the epidemic size and death of ruminants due to the virus; (\(ix\)) delay in the administration of vaccine may lead to a larger epidemic and greater loss of ruminant population as compared to no delay; and (\(x\)) if vaccination rate depends on time, a maximal attempt of vaccination at the beginning before an outbreak starts is suggested, and in such an event it may be possible to reduce the vaccination rate later during enzootic periods.
Vaccination against RVFV has been used for many years in Africa to reduce economic losses especially in highly susceptible breeds of sheep and cattle (Davies 2010) and prediction (\(viii\)) also supports implementation of this intervention. Delay in the administration of vaccine has been suggested to have serious consequences and is often linked to slow detection, laboratory confirmation, and limited vaccination resources due to infrequent outbreaks (McElroy et al. 2009). In light of prediction (\(ix\)), delay in vaccination may lead to inefficient RVFV control which may cause a more serious outbreak and greater loss of ruminants and if the vaccination campaign is withheld too long it may lead to outcomes similar to no vaccination. As remote sensing satellite imagery has been used to help predicting areas where outbreaks of RVF are expected to occur and enhance various preparedness activities (Anyamba et al. 2009, 2010), predictions (\(ix\))–(\(x\)) indicate that satellite RVF risk mapping forecasting may be an important key to implement controls efficiently.
We believe that the particular work considered here gives certain useful insights in understanding the transmission dynamics of RVFV, the transition between epizootic and enzootic cycles, and the use of vaccination to control the spread of RVFV. We also believe that it helps to identify the key variables and make predictions that can be further investigated in the field.
References
Abdo-Salem S, Waret-Szkuta A, Roger F, Olive M, Saeed K, Chevaller V (2011) Risk assessment of the introduction of Rift Valley fever from the Horn of Africa to Yemen via legal trade of small ruminants. Trop. Anim. Health Prod. 43:471–480
Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9:467–487
Andriamandimby SF, Randrianarivo-Sologoniaina AE, Jeanmaire EM, Ravololomanana L, Razafimanantsoa LT et al (2010) Rift Valley fever during rainy seasons, Madagascar, 2008–2009. Emerg. Infect. Dis. 16:963–970
Anyamba A, Chretien J, Small J, Tucker CJ, Formenty PB, Richardson JH, Britch SC, Schnabel DC, Erickson RL, Erickson RL (2009) Prediction of a Rift Valley fever outbreak. Proc. Natl. Acad. Sci. USA 106:955–959
Anyamba A, Linthicum KJ, Mahoney R, Tucker CJ, Kelley PW (2002) Mapping potential risk of rift valley fever outbreaks in African savannas using vegetation index time series data. Photogramm. Eng. Rem. S 68:137–145
Anyamba A, Linthicum KJ, Small J, Britch SC, Pak E et al (2010) Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006–2008 and possible vector control strategies. Am. J. Trop. Med. Hyg. 83:43–51
Balkhy HH, Memish ZA (2003) Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int. J. Antimicrob. Agents 21:153–157
Barker CM, Niu T, Reisen WK, Hartley DM (2013) Data-driven modeling to assess receptivity for Rift Valley fever virus. PLoS Negl. Trop. Dis. 7:e2515. doi:10.1371/journal.pntd.0002515
Barnard BJH (1979) Rift Valley fever vaccine-antibody and immune response in cattle to a live and an inactivated vaccine. J. S. Afr. Vet. Assoc. 50:155–157
Bicout DJ, Sabatier P (2004) ‘Mapping Rift Valley fever vectors and prevalence using rainfall variations’. Vector-borne Zoonot Dis. 4:33–42
Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ (2009) Rift Valley fever virus. J. Am. Vet. Med. Assoc. 234:883–893
Canyon DV, Hii JLK, Muller R (1999) The frequency of host biting and its effect on oviposition and survival in Aedes aegypti (Diptera: Culicidae). B. Entomol. Res. 89:35–39
Chevalier V, Rakotondrafara T, Jourdan M, Heraud JM, Andriamanivo HR, Durand B, Ravaomanana J, Rollin PE, Rakotondravao R (2011) An unexpected recurrent transmission of Rift Valley fever virus in cattle in a temparate and mountainous area of Madagascar. PLoS Neglect. Trop. Dis. 5:e1423. doi:10.1371/journal.pntd.0001423
Childs DZ, Boots M (2010) The interaction of seasonal forcing and immunity and the resonance dynamics of malaria. J. R. Soc. Interface 7:309–319
Chitnis N, Hyman JM, Manore CA (2013) Modelling vertical transmission in vectorborne diseases with applications to Rift Valley fever. J. Biol. Dynam. 7:11–40
Daubney R, Hudson JR, Garnham PC (1931) Enzootic hepatitis or RVF: an undescribed virus disease of sheep, cattle and man from East Africa. J. Pathol. Bacteriol. 34:545–579
Davies FG (2006) Risk of a Rift Valley fever epidemic at the haj in Mecca, Saudi Arabia. Rev. sci. tech. Off. int. Epiz. 25:137–147
Davies FG (2010) The historical and recent impact of Rift Valley fever in Africa. Am. J. Trop. Med. Hyg. 83:73–74
Davies FG, Kilelu E, Linthicum KJ, Pegram RG (1992) Patterns of Rift Valley fever virus. Epidemiol. Infect. 108:185–191
Diekmann O, Heesterbeek JAP, Roberts MG (2010) The construction of next-generation matrices for compartmental epidemic models. J. Royal Soc. Interface 7:873–885
Evans A, Gakuya F, Paweska JT, Rostal M, Akoolo L et al (2008) Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol. Infect. 136:1261–1269
Favier C, Chalvet-Monfray K, Sabatier P, Lancelot R, Fontenille D, Dubois MA (2006) Rift Valley fever in West Africa: the role of space in endemicity. Trop. Med. Int. Health 11:1878–1888
Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP, Zeller HG (1998) New vectors of Rift Valley fever in West Africa. Emerg. Infect. Dis. 4:289–293
Freier JE, Rosen L (1987) Vertical transmission of dengue virus by the mosquitoes of the Aedes scutellaris group. Am. J. Trop. Med. Hyg. 37:640–647
Gad AM, Feinsod FM, Allam IH, Eisa M, Hassan AN, Soliman BA, el Said S, Saah AJ (1986) A possible route for the introduction of Rift Valley fever virus into Egypt during 1977. J. Trop. Med. Hyg. 89:233–236
Gaff H, Hartley DM, Leahy NP (2007) (2007), ‘An epidemiological model of Rift Valley fever’ . Electron. J. Differ. Equ. 115:1–12
Gage KL, Burkot TR, Eisen RJ, Hayes EB (2008) Climate and vectorborne diseases. Am. J. Prev. Med. 35:436–450
Gao D, Cosner C, Cantrell RS, Beier JC, Ruan S (2013) Modeling the spatial spread of Rift Valley fever in Egypt. Bull. Math. Biol. 75:523–542
Gupta S, Swinton J, Anderson RM (1994) Theoretical studies of the effects of heterogeneity in the parasite population on the transmission dynamics of malaria. Proc. R. Soc. Lond. B 256:231–238
Hollidge BS, Gonzalez-Scarano F, Soldan SS (2010) Arboviral encephalitides: transmission, emergence, and pathogenesis. J. Neuroimmune. Pharm. 5:428–442
Ikegami T, Makino S (2009) Rift Valley fever vaccines. Vaccine 27:D69–D72
Kasari TR, Lynn TV, Weaver JT (2008) Evaluation of pathways for release of Rift Valley fever virus into domestic ruminant livestock, ruminant wildlife, and human populations in the continental United States. J. Am. Vet. Med. Assoc. 232:514–529
Keeling MJ and Rohani, P (2007) . Princeton University Press, Modeling Infectious Diseases in Humans and Animals
Kortekaas J, de Boer SM, Kant J, Vloet RPM, Antonis AFG, Moormann RJM (2010) Rift Valley fever virus immunity provided by a paramyxovirus vaccine vector. Vaccine 28:4394–4401
Lenhart S, Workman JT (2007) Optimal Control Applied to Biological Models. Chapman & Hall/CRC, London
Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ (1999) Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 285:397–400
Linthicum KJ, Davies FG, Kairo A, Bailey CL (1985) Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). isolations from diptera collected during an inter-epizootic period in kenya. J. Hyg. (Lond) 95:197–209
Majok AA, Zessin KH, Baumann MPO, Farver TB (1991) Analyses of baseline survey data on rinderpest in Bahr el Ghazal Province, with proposal of an improved vaccination strategy against rinderpest for southern Sudan. Trop. Anim. Health Prod. 23:186–196
Manore, C. A. and Beechler, B. R. (2013), Inter-epidemic and between-season persistence of Rift Valley fever: vertical transmission or cryptic cycling?, Transbound. Emerg. Dis. pp. 11. doi:10.1111/tbed.12082.
McElroy AK, Albarino CG, Nichol ST (2009) Development of a RVFV ELISA that can distinguish infected from vaccinated animals. Virol. J. 6:125
Métras R, Collins LM, White RG, Alonso S, Chevalier V, Thuranira-McKeever C, Pfeiffer DU (2011) ‘Rift Valley fever epidemiology, surveillance, and control: what have models contributed?’, Vector-Borne Zoonot Dis. 11:761–771
Mpeshe SC, Haario H, Tchuenche JM (2011) A mathematical model of Rift Valley fever with human host. Acta Biotheor. 59:231–250
Murithi RM, Munyua P, Ithondeka PM, Macharia JM, Hightower A, Luman ET, Breiman RF, Njenga MK (2011) Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts. Epidemiol. Infect. 139:372–380
Niu T, Gaff HD, Papelis YE, Hartley DM (2012) An epidemiological model of Rift Valley fever with spatial dynamics. Comput. Math. Methods Med. 2012:12. doi:10.1155/2012/138757
Paweska JT, Mortimer E, Leman PA, Swanepoel R (2005) An inhibition enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley virus in humans, domestic and wild ruminants. J. Virol. Methods 127:10–18
Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J (2010) Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagonostics and prevention. Vet. Res. 41:61
Reiskind MH, Westbrook CJ, Lounibos LP (1987) Exposure to Chikungunya virus and adult longevity in Aedes aegypti (l.) and Aedes albopictus (skuse). J. Vector. Ecol. 37:640–647
Rostal MK, Evans AL, Sang R, Gikundi S, Wakhule L, Munyua P, Macharia J, Feikin DR (2010) Identification of potential vectors and detection of antibodies against Rift Valley fever virus in livestock during interepizootic periods. Am. J. Vet. Res. 71:522–526
Swanepoel R, Struthers JK, Erasmus MJ, Sheperd SP, McGillivray GM, Shepherd AJ, Hummitzsch DE, Erasmus BJ, Barnard BJ (1986) Comparative pathogenicity and antigenic cross-reactivity of Rift Valley fever and other African phleboviruses in sheep. J. Hyg. (Lond) 97:331–346
Thiongane Y, Gonzalez JP, Fati A, Akakpo JA (1997) Changes in Rift Valley fever neutralizing antibody prevalence among small domestic ruminants following the 1987 outbreak in the Senegal River basin. Res. Virol. 142:67–70
Turell MJ, Kay BH (1998) Susceptibility of selected strains of Australian mosquitoes (Diptera: Culicidae) to Rift Valley fever virus. J. Med. Entomol. 35:132–135
Turell MJ, Linthicum KJ, Patrican LA, Davies FG, Kairo A, Bailey CI (2008) Vector competence of selected African mosquito (Diptera: Culicidae) species for Rift Valley fever virus. J. Med. Entomol. 45:102–108
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 285:29–48
Weaver SC, Reisen WK (2010) Present and future arboviral threats. Antivir. Res. 85:328–345
Xue L, Cohnstaedt LW, Scott HM, Scoglio C (2013) A hierarchical network approach for modeling Rift Valley fever epidemics with applications in North America. PLoS ONE 8:e62049. doi:10.1371/journal.pone.0062049
Xue L, Scott HM, Cohnstaedt LW, Scoglio C (2012) A network-based meta-population approach to model Rift Valley fever epidemics. J. Theoret. Biol. 306:129–144
Acknowledgments
This work was supported by the National Institute of Health (NIH) grant R01GM093345.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chamchod, F., Cantrell, R.S., Cosner, C. et al. A Modeling Approach to Investigate Epizootic Outbreaks and Enzootic Maintenance of Rift Valley Fever Virus. Bull Math Biol 76, 2052–2072 (2014). https://doi.org/10.1007/s11538-014-9998-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-9998-7