Abstract
Gonadotropin-releasing hormone (GnRH) neurons have two major processes that have properties of both dendrites (they receive synaptic input from other neurons) and axons (they actively propagate action potentials to the synaptic terminal). These processes have thus been termed dendrons. We construct a stochastic spatiotemporal model of the dendron of the GnRH neuron, with the goal of studying how stochastic synaptic input along the length of the dendron affects the initiation and propagation of action potentials. We show (1) that synaptic inputs closer to the soma are effective controllers of action potential initiation and electrical bursting and (2) that although the effects on the amplitude and width of propagating action potentials are critically dependent on the timing and location of synaptic input addition, the effects remain small. We conclude that although stochastic synaptic input along the length of the dendron is likely to be a major determinant of action potential initiation, it is an unlikely mechanism for controlling whether or not action potentials reach the synaptic terminal. Thus, the role of synaptic inputs situated along the dendron a long way from the site of action potential initiation remains unclear. We also show that the actions of kisspeptin can result in significant modulation of the amount of calcium released by an action potential at the synaptic terminal. Furthermore, we show that the actions of kisspeptin are greatest when multiple effects operate together and that a kisspeptin-induced increase in firing rate is, by itself, less effective at increasing \(\mathrm{Ca}^{2+}\) release than is a combination of an increased firing rate, an increase in \(\mathrm{Ca}^{2+}\) influx, and an increase in inositol trisphosphate (\(\mathrm{IP}_3\)) production. We conclude that the inherent synergies in the various actions of kisspeptin make it a likely candidate for the precise control of \(\mathrm{Ca}^{2+}\) transients at the synaptic terminal.






Similar content being viewed by others
References
Brailoiu GC, Dun SL, Ohsawa M, Yin D, Yang J, Chang JK, Brailoiu E, Dun NJ (2005) KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol 481:314–329
Campbell RE, Han SK, Herbison AE (2005) Biocytin filling of adult gonadotropin releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology 146:1163–1169
Campbell RE, Gaidamaka G, Han SK, Herbison AE (2009) Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc Natl Acad Sci USA 106:10835–10840
Castellano JM, Navarro VM, Fernández-Fernández R, Castaño JP, Malagón MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M (2006) Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol 257–258:75–83
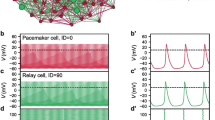
Chen X, Iremonger K, Herbison A, Kirk V, Sneyd J (2013) Regulation of electrical bursting in a spatiotemporal model of a GnRH neuron. Bull Math Biol 75:1941–1960
Christian CA, Moenter SM (2008) Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology 149(6):3130–3136
Chu Z, Takagi H, Moenter SM (2010) Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci 30(40):13373–13383
Clasadonte J, Poulain P, Beauvillain J, Prevot V (2008) Activation of neuronal nitrix oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology 149(2):587–596
Constantin S, Caligioni CS, Stojilkovic S, Wray S (2009) Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology 150(3):1400–1412
Cottrell EC, Campbell RE, Han S, Herbison AE (2006) Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 147(8):3652–3661
d’Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH (2007) Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 104(25):10714–10719
d’Anglemont de Tassigny X, Fagg LA, Carlton MBL, Colledge WH (2008) Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149(8):3926–3932
de Roux N, Genin E, Carel J, matsuda F, Chaussain J, Milgrom E (2003) Hypogonadotropic hypogonadism due to loss of function of the Kiss1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100(19):10972–10976
Gerhold LM, Rosewell KL, Wise PM (2005) Suppression of vasoactive intestinal polypeptide in the suprachiasmatic nucleus leads to aging-like alterations in cAMP rhythms and activation of gonadotropin-releasing hormone neurons. J Neurosci 25(1):62–67
Gerhold LM, Wise PM (2006) Vasoactive intestinal polypeptide regulates dynamic changes in astrocyte morphometry: impact on gonadotropin-releasing hormone neurons. Endocrinology 147(5):2197–2202
Gin E, Falcke M, Wagner LE II, Yule DI, Sneyd J (2009) A kinetic model of the inositol trisphosphate receptor based on single-channel data. Biophys J 96:4053–4062
Gottsch ML, Clifton DK, Steiner RA (2006) Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol 254–255:91–96
Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA (2004) A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145(9):4073–4077
Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD (2002) Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol 87:2287–2296
Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE (2005) Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25(49):11349–11356
Herbison AE (2006) Physiology of the GnRH neuronal network. In: Neill JD (ed) Knobil and Neill’s physiology of reproduction, 3rd edn. Academic Press, San Diego, pp 1415–1482
Herbison AE, Moenter SM (2011) Depolarising and hyperpolarising actions of \(\text{GABA}_{{\rm A}}\) receptor activation on gonadotrophin-releasing hormone neurones: towards and emerging consensus. J Neuroendocrinol 23:557–569
Herde MK, Iremonger KJ, Constantin S, Herbison AE (2013) GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci 33(31):12689–12697
Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA (2004) Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuronendocrinology 80:264–272
Iremonger KJ, Constantin S, Liu X, Herbison AE (2010) Glutamate regulation of GnRH neuron excitability. Brain Res 1364:35–43
Iremonger KJ, Herbison AE (2012) Initiation and propagation of action potentials in GnRH neuron dendrites. J Neurosci 32(1):151–158
Jackson MB, Konnerth A, Augustine GJ (1991) Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA 88:380–384
Keen KL, Wenger FH, Bloom SR, Ghatei MA, Terasawa E (2008) An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149(8):4151–4157
Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schütz G, Herbison AE (2013) Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun 4(2492): doi:10.1038/ncomms3492
Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden J, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandepun F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M (2001) The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276(37):34631–34636
Kroll H, Bolsover S, Hsu J, Kim S, Bouloux P (2011) Kisspeptin-evoked calcium signals in isolated primary rat gonadotropin-releasing hormone neurones. Neuroendocrinology 93:114–120
Lee K, Duan W, Sneyd J, Herbison AE (2010) The slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci 20(18):6214–6224
Liu X, Lee K, Herbison AE (2008) Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149:4605–4614
Messager S, Chatzidaki EE, Ma D, hendrick AG, Zahn D, Dixon J, Thresher RR, malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR (2005) Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102(5):1761–1766
Palk L, Sneyd J, Shuttleworth TJ, Yule D, Crampin E (2010) A dynamic model of saliva secretion. J Theor Biol 266(4):625–640
Pielecka-Fortuna J, Chu Z, Moenter SM (2008) Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149(4):1979–1986
Roberts CB, Campbell RE, Herbison AE, Suter KJ (2008) Dendritic action potential initiation in hypothalamic gonadotropin-releasing hormone neurons. Endocrinology 149:3355–3360
Sasaki T, Matsuki N, Ikegaya Y (2011) Action-potential modulation during axonal conduction. Science 331:599
Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, OŔahilly S, Calton MBL, Crowley WF, Aparicio SAJR, Colledge WH (2003) The GPR54 gene as a regulator of puberty. N Engl J Med 349:27–1614
Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ (2011) Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Neuroendocrinology 152(3):1001–1012
Stafford LJ, Xia C, Ma W, Cai Y, Liu M (2002) Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res 62:5399–5404
Stuart GJ, Häusser M (2001) Dendritic coincidence detection of EPSPs and action potentials. Nat Neurosci 4:63–71
Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, Ajiki K, Ichikawa M, Okamura H, Maeda K-I, Tsukamura H (2011) Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol 23:863–870
Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ (2013) Kisspeptin activation of TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology 154(8):2772–2783
Zhang C, Rønnekleiv OK, Kelly MJ (2013) Kisspeptin inhibits a slow afterhyperpolarization current via protein kinase C and reduces spike frequency adaptation in GnRH neurons. Am J Physiol Endocrinol Metab 304:E1237–E1244
Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK (2008) Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28(17):4423–4434
Zhang X, Spergel D (2012) Kisspeptin inhibits high-voltage activated \(\text{Ca}^{2+}\) channels in GnRH neurons via multiple \(\text{Ca}^{2+}\) influx and release pathways. Neuroendocrinology 96:68–80
Acknowledgments
We thank Dr Karl J. Iremonger and Prof. Allan E. Herbison for their helpful comments. This work was supported by the New Zealand Health Research Council and by a University of Auckland Doctoral Scholarship to Xingjiang Chen. The author(s) wish to acknowledge the contribution of the NeSI high-performance computing facilities and the staff at the Centre for eResearch at the University of Auckland. New Zealand’s national facilities are provided by the New Zealand eScience Infrastructure (NeSI and funded jointly by NeSI’s collaborator institutions and through the Ministry of Business, Innovation and Employment’s Infrastructure programme. http://www.nesi.org.nz.
Author information
Authors and Affiliations
Corresponding author
Appendix: The deterministic model of a GnRH neuron introduced in Chen et al. 2013
Appendix: The deterministic model of a GnRH neuron introduced in Chen et al. 2013
1.1 Voltage Submodel
The equation for membrane potential (\(V\)) in the voltage subsystem is
where \(C_\mathrm{m}\) is the membrane capacitance and \(I_\text {ionic} \) is the sum of the ionic currents.
The soma is the region \([0, x_1]\), the dendrite between the soma and the iSite is \([x_1, x_2]\), the iSite is \([x_2, x_3]\), and the dendrite elsewhere is \([x_3, x_4]\).
For \(x \in [0, x_1]\), the currents in the soma are modeled as
For \(x \in [x_2, x_3]\), the currents in the iSite are the same as in the soma, except that we use a higher conductance for \(I_\text {naf}\), representing a higher density of \(\mathrm{Na}^{+}\) channels in the iSite. We use a \(\mathrm{Na}^{+}\) conductance (\(g_\text {naf}\)) of 410 nS in the iSite, and 150 nS elsewhere (Table 2).
For \(x \in [x_1, x_2]\) and \(x \in [x_3, x_4]\), the currents in the dendrite are modeled as
\(I_\text {naf}\) and \(I_\text {nap}\) denote the fast, persistent \(\mathrm{Na}^{+}\) currents, \(I_\text {kdr}\), \(I_\text {kir}\), and \(I_\text {km}\) denote the delayed rectifier, inward rectifier, and m-type \(\mathrm{K}^{+}\) currents, respectively, \(I_\text {cal}\) and \(I_\text {cat}\) are L-type and T-type \(\mathrm{Ca}^{2+}\) currents, \(sI_\text {AHP-SK}\) is an SK-type \(\mathrm{Ca}^{2+}\)-activated \(\mathrm{K}^{+}\) current, and \(sI_\text {AHP-UCL}\) is a slow \(\mathrm{Ca}^{2+}\)-activated afterhyperpolarization current. \(I_\text {App}\) is a passive membrane leakage current. It may incorporate current from synaptic inputs, although there are no explicit synaptic inputs in our model. All the ion channels and fluxes are modeled as in Lee et al. (2010) and references therein.
We used a Hodgkin–Huxley formalism to model the currents. For example, \(I_\mathrm{naf}\) is described as
where \(g_\text {naf}\) is the maximum conductance, \(M_\text {naf}\) is the activation gating variable, \(H_\text {naf}\) is the inactivation gating variable, and \(V_\text {na}\) is the reversal potential for \(\mathrm{Na}^{+}\). Similarly, equations governing the other voltage-dependent currents are described by
The gating variables \(M_\text {naf}, M_\text {nap}, N_\text {kir}, M_\text {cal}, M_\text {cat}\), and \(H_\text {cat}\) are set to their steady-state values, while the gating variables \(H_\text {naf}, N_\text {kdr}\), and \(N_\text {km}\) are modeled by
The steady-state functions \(H_\text {naf}, N_\text {kdr}\), and \(N_\text {km}\) can be found in Lee et al. (2010).
The equation for \(sI_\text {AHP-SK}\) is
The equation for \(sI_\text {AHP-UCL}\) is
where \(O_\text {ucl}\) and \(O^*_\text {ucl}\) are two open states of the channel governed by the kinetic equations of the system introduced in Lee et al. (2010).
1.2 Calcium Submodel
The equations describing the calcium concentration in the cytosol \((c)\) and in the endoplasmic reticulum (ER)\((c_\mathrm{e})\) are as follows:
where \(\rho \) is used to scale plasma membrane and ER fluxes, and \(\gamma \) is the volume ratio between the ER and the cytosol. \(J_\text {in}\), \(J_\text {pm}\), \(J_\text {release}\), and \(J_\text {serca}\) denote the influx via plasma membrane channels, efflux via PMCA and NCX plasma membrane pumps, release of \(\mathrm{Ca}^{2+}\) from the ER to cytosol, and \(\mathrm{Ca}^{2+}\) pumping from the cytosol to the ER, respectively. We have
The IPR open probability \((P_\mathrm{o})\) is from Gin et al. (2009):
where \(q_{12}, q_{21}, q_{24}\), and \(q_{42}\) are set to their steady-state values, and where \(q_{23}\) and \(q_{32}\) are given by
Since \(\mathrm{Ca}^{2+}\) diffusion is orders of magnitude slower than the diffusion of \(V\), \(\mathrm{Ca}^{2+}\) diffusion was omitted from all our model simulations.
Rights and permissions
About this article
Cite this article
Chen, X., Sneyd, J. A Computational Model of the Dendron of the GnRH Neuron. Bull Math Biol 77, 904–926 (2015). https://doi.org/10.1007/s11538-014-0052-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-0052-6