Abstract
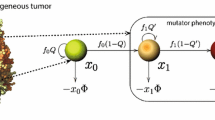
Genetic instability promotes cancer progression (by increasing the probability of cancerous mutations) as well as hinders it (by imposing a higher cell death rate for cells susceptible to cancerous mutation). With the loss of tumor suppressor gene function known to be responsible for a high percentage of breast and colorectal cancer (and a good fraction of lung cancer and other types as well), it is important to understand how genetic instability can be orchestrated toward carcinogenesis. In this context, this paper gives a complete characterization of the optimal (time-varying) cell mutation rate for the fastest time to a target cancerous cell population through the loss of both copies of a tumor suppressor gene. Similar to the (one-step) oncogene activation model previously analyzed, the optimal mutation rate of the present two-step model changes qualitatively with the convexity of the (mutation rate-dependent) cell death rate. However, the structure of the Hamiltonian for the new model differs significantly and intrinsically from that of the one-step model, and a completely new approach is needed for the solution of the present two-step problem. Considerable insight into the biology of optimal switching (between corner controls) is extracted from numerical results for cases with nonconvex death rates.
Similar content being viewed by others
References
Artandi SE, DePinho RA (2000) A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev 10(1):39–46
Ashworth A, Lord CJ, Reis-Filho JS (2011) Genetic interactions in cancer progression and treatment. Cell 145:30–38
Bailey SM, Murnane JP (2006) Telomeres, chromosome instability and cancer. Nucleic Acids Res 34(8):2408–2417
Bardelli A et al (2001) Carcinogen-specific induction of genetic instability. PNAS 98:5770–5575
Bartek J (2011) DNA damage response, genetic instability and cancer: from mechanistic insights to personalized treatment. Mol Oncol 5(4):303–307
Bartkova J et al (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434(7035):864–870
Branzei D, Foiani M (2010) Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol 11:208–219
Breivik J (2005) The evolutionary origin of genetic instability in cancer development. Semin Cancer Biol 15(1):51–60
Bristow RG, Hill RP (2008) Hypoxia and metabolism: hypoxia, DNA repair and genetic instability. Nat Rev Cancer 8(3):180–192
Bryson A, Ho YC (1969) Applied optimal control. Ginn and Company, Waltham, MA
Cahill DP et al (1999) Genetic instability and Darwinian selection in tumours. Trends Cell Biol 9(12):57–60
Campbell CD, Chong JX, Malig M et al (2012) Estimating the human mutation rate using autozygosity in a founder population. Nat Genet 44(11):1277–1281
Corcos D (2012) Unbalanced replication as a major source of genetic instability in cancer cells. AJBR 2(3):160–169
Chin K et al (2004) In situ analyses of genome instability in breast cancer. Nat Genet 36(9):984–988
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30(7):1073–1081
Czernilofsky AP, Levinson AD, Varmus HE, Bishop JM, Tischer E, Goodman HM (1980) Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature 287:198–203
de Pillis LG, Radunskaya AE (2003) The dynamics of an optimally controlled tumor model: a case study. Math Comput Model 37(11):1221–1244
de Pillis LG et al (2007) Chemotherapy for tumors: an analysis of the dynamics and a study of quadratic and linear optimal controls. Math Biosci 209:291–315
dePillis LG et al (2007) Seeking bang–bang solutions of mixed immuno-chemotherapy of tumors. Elec EJDE 171:1–24
Deng C-X (2006) BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res 34(5):1416–1426
Gelfand IM, Fomin SV (1963) Calculus of variations. Prentice Hall, Englewood Cliffs NJ
Gill P, Murrray W, Saunders MA (2005) SNOPT: an SQP algorithm for large-scale constrained optimization problem. SIAM Rev 47:99–131
Gonzalez-Suarez E et al (2000) Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat Genet 26(1):114–117
Gorgoulis VG et al (2005) Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434(7035):907–913
Grady WM, Carethers JM (2008) Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 135(4):1079–1099
Kinzler K, Vogelstein B (2002) The genetic basis of human cancer, 2nd edn. The McGraw-Hill Companies Inc, New York
Kirschner D, Lenhart S, Serbin S (1997) Optimal control of the chemotherapy of HIV. J Math Biol 35(7):775–792
Knudson AG (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci 68(4):820–823
Komarova NL et al (2002) Dynamics of genetic instability in sporadic and familial colorectal cancer. Cancer Biol Ther 1(6):685–692
Komarova NL, Sengupta A, Nowak MA (2003) Mutation-selection networks of cancer initiation: tumor suppressor genes and chromosomal instability. J Theor Biol 223(4):433–450
Komarova NL, Wodarz D (2004) The optimal rate of chromosome loss for the inactivation of tumor suppressor genes in cancer. Proc Natl Acad Sci USA 101(18):7017–7021
Komarova N (2004) Does cancer solve an optimization problem? Cell Cycle 3(7):840–844
Komarova NL, Sadovsky A, Wan FYM (2008) Selective pressures for and against genetic instability in cancer: a time-dependent problem. J R Soc Interface 5:105–121
Ledzewicz U et al (2013) Optimal controls for a mathematical model of tumor-immune interactions under targeted chemotherapy with immune boost. DCDS-B 18(4):1013–1051
Lengauer C, Kinzler KW, Vogelstein B (1997) Genetic instability in colorectal cancers. Nature 386(6625):623–627
Lengauer C, Kinzler KW, Vogelstein B (1998) Genetic instabilities in human cancers. Nature 396(6712):643–649
Lenhart S, Workman JT (2007) Optimal control applied to biological models. Chapman and Hall/CRC, London
Loeb LA, Springgate CF, Battula N (1974) Errors in DNA replication as a basis for malignant changes. Cancer Res 34:2311–2321
Martin GS (2001) The hunting of the Src. Nat Rev Mol Cell Biol 2:467–475
Maslov AY, Vijg J (2009) Genome instability, cancer and aging. Biochimica et Biophysica Acta (BBA) General Subjects 1790(10):963–969
Michor F et al (2005) Can chromosomal instability initiate tumorigenesis? Semin Cancer Biol 15(1):43–49
Negrini S, Gorgoulis VG, Halazonetis TD (2010) Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol 11:220–228
Nowak MA, Michor F, Iwasa Y (2006) Genetic instability and clonal expansion. J Theor Biol 241(1):26–32
Nouspikel T (2013) Genetic instability in human embryonic stem cells: prospects and caveats. Future Oncol 9(6):867–877
Perucho M (1996) Cancer of the microsatellite mutator phenotype. Biol Chem 377:675–684
Pikor L, Thu K, Vucic E, Lam W (2013) The detection and implication of genome instability in cancer. Cancer Metastasis Rev 32(3–4):341–352
Pontryagin LS et al (1962) The mathematical theory of optimal control processes. Interscience Publishers, New York
Rudolph KL et al (2001) Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet 28(2):155–159
Samper E, Flores JM, Blasco MA (2001) Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc\(^{-/-}\) mice with short telomeres. EMBO Rep 2(9):800–807
Sanchez-Tapia C (2015) Ph.D. Dissertation, Mathematics, UC Irvine
Shih IM et al (2001) Evidence that genetic instability occurs at an early stage of colorectal tumorigenesis. Cancer Res 61(3):818–822
Smith LE et al (2003) Radiation-induced genomic instability: radiation quality and dose response. Health Phys 85(1):23–29
Swan GW (1990) Role of optimal control theory in cancer chemotherapy. Math Biosci 101(2):237–284
Wan FYM (1995) Introduction to the calculus of variations and its applications. Chapman and Hall, New York
Wan FYM, Sadovsky A, Komarova NL (2010) Genetic instability in cancer: an optimal control problem. Stud Appl Math 125(1):1–10
Wodarz D, Komarova NL (2005) Computational biology of cancer: lecture notes and mathematical modeling. World Scientific, Singapore
Wodarz D, Komarova NL (2014) Dynamics of cancer: mathematical foundations of oncology. World Scientific, Singapore
Acknowledgments
The research was supported by NIH Grants R01-GM067247, P50-GM076516 and, for the first author, also a UC MEXUS-CONACYT Fellowship from Mexico and the University of California Institute for Mexico and the USA. The NIH R01 grant was awarded through the Joint NSF/NIGMS Initiative to Support Research in the Area of Mathematical Biology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Also member of the NIH Center for Complex Biological System (CCBS) at the University of California, Irvine, and the Center for Mathematical and Computational Biology (CMCB) in its School of Physical Sciences.
Appendix
Appendix
1.1 Cell Populations for Upper Corner Control
With the two corner controls playing a key role in the optimal solution, we note that the state equations admit an exact solution for these controls. For the upper corner control \(u_{1}(t)=1\), the state equations simplify to
The following exact solutions for these uncoupled first-order state equations are immediate.
Lemma 49
For \(u(t)=u_{1}(t)\equiv 1\) and a general set of initial conditions \(x_{k}^{(1)}(T_{i})=x_{k}^{(i)}\ge 0,\,k=0,1,2\), the exact solution of the three uncoupled first-order state Eq. (82) is
with \(\tau =t-T_{i}\) and a superscript “\((1)\)” for upper corner control. The normalized mutated cell populations \(x_{1}^{(1)}\) and \(x_{2}^{(1)}\) are positive function of time for \(\tau =t-T_{i}>0\).
Remark 50
The concavity of \(x_{2}^{(1)}(t)\) also follows from
Remark 51
For the interval adjacent to the initial time \(t=T_{i}=0\), the exact solutions (83) and (84) simplify by the known initial conditions to
1.2 Cell Populations for Lower Corner Control
For the lower corner control \(u_{0}(t)=0\), the first two state Eqs. (1) and (2) simplify to
They may be solved exactly (by Mathematica or Maple) allowing for the satisfaction of two initial conditions
Instead of writing down the exact solution, we need only the following simple bounds on \(x_{0}^{(0)}\) and \(x_{1}^{(0)}\) for our purposes:
given the nonnegativity of the three cell population and \(0\le x_{0}^{(0)}+x_{1}^{(0)}\le 1\) by Lemma 4. It is possible to simplify the upper bound for \(dx_{1}^{(0)}/dt\) further by replacing the right-hand side of (91) with \(2\mu x_{0}^{(0)}+x_{1}^{(0)}\left( 1-\mu \right) \). We refrain from doing so to get sharper results.
Lemma 52
where
Proof
The various inequalities are straightforward consequences of the inequalities (90) and (91) along with the switch conditions (89): In particular, we have
where
and
keeping in mind \(x_{1}^{(s)}<1<x_{1}^{(p)}\). \(\square \)
Remark 53
We note that the upper bound for \(x_{1}^{(0)}\) is unrealistic since \(x_{0}^{(0)}+x_{1}^{(0)}<1\) (by Lemma 1) and \(x_{0}^{(0)}\ge 0\). However, \(x_{1}^{(p)}\lesssim 1+\mu \) is only an (overly conservative) upper bound and does not contradict other more realistic results for cell populations (such as Lemma 1).
For \(u=0\), the third state Eq. (3) takes the form
which is a linear first-order ODE for the only unknown \(x_{2}^{(0)}\) and can be solved with the help of an integrating factor. Even without the explicit solution, we see from (97) that \(x_{2}(t)\) increases without bound as \(t \rightarrow \infty \) since \(a\gg 1\).
More useful for our analysis is the following upper and lower bound for \(x_{2}^{(0)}\):
Lemma 54
The following inequalities hold for \(u=0\) and \(x_{2}^{(0)}(t=T_{\mathrm{s}})=x_{2}^{(s)}\):
Proof
With \(0\le x_{0}^{(0)}+x_{1}^{(0)}\le 1\) by Lemma 1, the ODE (97) implies
from which we get
\(\square \)
Rights and permissions
About this article
Cite this article
Sanchez-Tapia, C., Wan, F.Y.M. Fastest Time to Cancer by Loss of Tumor Suppressor Genes. Bull Math Biol 76, 2737–2784 (2014). https://doi.org/10.1007/s11538-014-0027-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-0027-7