Abstract
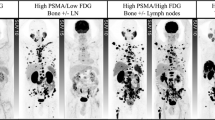
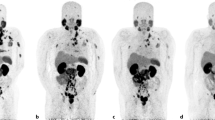
Nanomedicine allows achievement of tumor-selective drug delivery because of the enhanced permeability and retention (EPR) effect of solid tumors. We report here the first clinical application of a new agent—HPMA copolymer-conjugated pirarubicin (P-THP)—with a molecular size of about 8 nm, or 38.5 kDa. A patient had advanced prostate cancer with multiple metastases in the lung, pelvis, femur, and perhaps the sacrum. In April 2013, this 60-year-old patient started treatment with leuprorelin and estradiol, which continued until July 2014, but the patient became refractory to this treatment. So the patient underwent proton beam radiotherapy targeted to the primary prostate cancer, and P-THP was administered for numerous metastatic tumor nodules concomitantly with radiotherapy. This combination therapy had remarkable results, with complete remission of multiple metastases in the lung and bone. The prostate-specific antigen (PSA) value was decreased from about 1000 ng/mL on April 30, 2013, to about 100 ng/mL on June 24, 2013, with hormone therapy, but rose again to 964.2 ng/mL and then to 1472 ng/mL in July 2013, during leuprorelin administration. P-THP treatment administered concomitantly with proton beam irradiation was started in August 2013. The PSA value was decreased to 102 ng/mL on August 26, 2013, and then to 0.971 ng/mL on October 8, 2013, and 0.277 ng/mL on January 15, 2015. The P-THP doses ranged from 30 to 75 mg of free THP equivalent/patient every 2–3 weeks without signs of serious toxicity, such as cardiovascular side effects or a reduction in quality of life. No evidence of relapse was found more than 20 months after P-THP administration. This case demonstrates the value of hydrazone-bonded polymeric drugs in multimodal therapy.



Similar content being viewed by others
Notes
EPR-effect: The enhanced permeability and retention (EPR) effect of solid tumors is a unique vascular pathophysiology observed in most solid tumors. It is due to defective vascular architecture and extensive production of vascular permeability factors in tumor tissues, such as nitric oxide, bradykinin, etc. See references [3–6].
References
Leaf C (2013) The truth in small doses: why we’re losing the war on cancer and how to win it. Simon & Schuster, New York
Nakamura H, Etrych T, Chytil P et al (2014) Two step mechanisms of tumor selective delivery of N-(2-hydroxypropyl)methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage. J Control Release 174:81–87
Maeda H, Nakamura H, Fang J (2013) The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev 65:71–79
Maeda H (2012) Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting. Proc Jpn Acad Ser B Phys Biol Sci 88:53–71
Nakamura H, Fang J, Maeda H (2015) Development of next-generation macromolecular drugs based on the EPR effect: challenges and pitfalls. Expert Opin Drug Deliv 12:53–64
Lammers T, Hennink WE, Storm G (2008) Tumor-targeted nanomedicines: principles and practice. Br J Cancer 99:392–397
Lammers T, Subr V, Peschke P et al (2008) Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. Br J Cancer 99:900–910
Tsukigawa K, Liao L, Nakamura H et al (2015) Synthesis and therapeutic effect of styrene-maleic acid copolymer conjugated pirarubicin. Cancer Sci 106:270–278
Daruwalla J, Nikfarjam M, Greish K et al (2010) In vitro and in vivo evaluation of tumor targeting styrene-maleic acid copolymer-pirarubicin micelles: survival improvement and inhibition of liver metastases. Cancer Sci 101:1866–1874
Daruwalla J, Greish K, Malcontenti-Wilson C et al (2009) Styrene maleic acid-pirarubicin disrupts tumor microcirculation and enhances the permeability of colorectal liver metastases. J Vasc Res 46:218–228
Daruwalla J, Greish K, Malcontenti-Wilson C, et al (2015) SMA-pirarubicin induces tumor-selective oxidative stress and decreases tumor hypoxia as possible treatment of colorectal cancer liver metastases. Surgery 158(1):236–47
Nakamura H, Fang J, Gahininath B et al (2011) Intracellular uptake and behavior of two types zinc protoporphyrin (ZnPP) micelles, SMA-ZnPP and PEG-ZnPP as anticancer agents; unique intracellular disintegration of SMA micelles. J Control Release 155:367–375
Nagai K, Nagasawa K, Ishimoto A et al (2003) Pirarubicin is taken up by a uridine-transportable sodium-dependent concentrative nucleoside transporter in Ehrlich ascites carcinoma cells. Cancer Chemother Pharmacol 51:512–518
Shelley M, Harrison C, Coles B, et al (2006) Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst Rev 18(4):CD005247. doi:10.1002/14651858.CD005247.pub2
Winquist E, Waldron T, Berry S et al (2006) Non-hormonal systemic therapy in men with hormone-refractory prostate cancer and metastases: a systematic review from the Cancer Care Ontario Program in Evidence-based Care’s Genitourinary Cancer Disease Site Group. BMC Cancer 6:112
Anderstrom C, Eddeland A, Folmerz P et al (1995) Epirubicin and medroxyprogesterone acetate versus estramustine phosphate in hormone-resistant prostatic cancer: a prospective randomized study. Eur Urol 27:301–305
Laurie JA, Hahn RG, Therneau TM et al (1992) Chemotherapy for hormonally refractory advanced prostate carcinoma. A comparison of combined versus sequential treatment with mitomycin C, doxorubicin, and 5-fluorouracil. Cancer 69:1440–1444
Stephens RL, Vaughn C, Lane M et al (1984) Adriamycin and cyclophosphamide versus hydroxyurea in advanced prostatic cancer. A randomized Southwest Oncology Group study. Cancer 53:406–410
Petrylak DP, Tangen CM, Hussain MH et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520
Berthold DR, Pond GR, Soban F et al (2008) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 26:242–245
Maeda H (2012) Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release 164:138–144
Seki T, Fang J, Maeda H (2009) Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci 100:2426–2430
Schmidt-Hansen M, Hoskin P, Kirkbride P et al (2014) Hormone and radiotherapy versus hormone or radiotherapy alone for non-metastatic prostate cancer: a systematic review with meta-analyses. Clin Oncol (R Coll Radiol) 26:e21–e46. doi:10.1016/j.clon.2014.06.016
van de Water TA, Bijl HP, Schilstra C et al (2011) The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist 16:366–377
Coen JJ, Zietman AL, Rossi CJ et al (2012) Comparison of high-dose proton radiotherapy and brachytherapy in localized prostate cancer: a case-matched analysis. Int J Radiat Oncol Biol Phys 82:e25–e31
Maeda H (2015) Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. doi:10.1016/j.addr.2015.01.002
Fang J, Liao L, Yin H, et al (2015) Photodynamic therapy based on tumor-targeted polymer-conjugated zinc protoporphyrin and irradiation with xenon light. Future Science OA FSO4. http://www.future-science.com/doi/pdf/10.4155/fso.15.2.
Kopecek J, Kopecková P (2010) HPMA copolymers: origins, early developments, present, and future. Adv Drug Deliv Rev 62:122–149
Ulbrich K, Subr V (2010) Structural and chemical aspects of HPMA copolymers as drug carriers. Adv Drug Deliv Rev 62:150–166
Acknowledgments
The authors thank Phillip Allen Suzuki for critical comments, A. Kovalik for technical assistance, and Judith B. Gandy for editing this paper. Thanks are also due for support from the Adaptable & Seamless Technology Transfer Program through Target-Driven R&D (A-STEP) Research Grants from the Government of Japan, a Grant-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science & Technology (MEXT; No. AS242Z01542Q), a Cancer Specialty Grant from the Japanese Ministry of Health, Welfare and Labor (MHWL), the H23-3rd Cancer Project-General-001 for HM, the Grant Agency of the Czech Republic (grant no. P207/12/J030), and the Ministry of Education, Youth and Sports of the Czech Republic (grant no. EE2.3.30.0029).
Conflict of Interest
None of the authors declared any conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dozono, H., Yanazume, S., Nakamura, H. et al. HPMA Copolymer-Conjugated Pirarubicin in Multimodal Treatment of a Patient with Stage IV Prostate Cancer and Extensive Lung and Bone Metastases. Targ Oncol 11, 101–106 (2016). https://doi.org/10.1007/s11523-015-0379-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-015-0379-4