Abstract
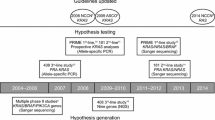
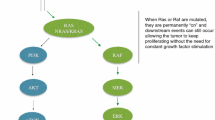
Targeted agents have become an integral part of the treatment of a number of malignant diseases and regimens containing agents that disrupt the epidermal growth factor receptor (EGFR) signaling pathway are now considered a standard therapeutic approach for a range of tumor types. Recently, the mutational status of the KRAS gene in tumors was shown to be predictive of outcome to treatment with EGFR-targeted therapies in metastatic colorectal cancer (mCRC). The immoglobulin (Ig) G1 EGFR-targeting monoclonal antibody (mAb), cetuximab, has been shown to provide significant clinical benefits when added to standard irinotecan- and oxaliplatin-containing chemotherapy regimens, first-line, in patients with KRAS wild-type mCRC. Its effects on tumor response and resectability of metastases make cetuximab a particularly useful treatment option for patients with bulky or initially unresectable disease. With an ever-increasing array of management options available, it is important that patients with mCRC receive the treatment that offers them the best chance of prolonged survival. In view of this, testing for tumor KRAS mutation status should be mandatory at diagnosis of mCRC, prior to treatment decision-making.
Similar content being viewed by others
References
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P, Mirtsching B, Cohn AL, Pippas AW, Azarnia N, Tsuchihashi Z, Mauro DJ, Rowinsky EK (2006) Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol 24:4914–4921
Folprecht G, Lutz MP, Schöffski P, Seufferlein T, Nolting A, Pollert P, Kohne CH (2006) Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol 17:450–456
Martinelli E, Macarulla T, Vega-Villegas E, Rodriguez-Braun E, Ciardiello F, Ramos F, Rivera F, Stroh C, Nippgen J, Cervantes A, Baselga J, Tabernero J (2008) First-line therapy with cetuximab followed by cetuximab plus FOLFIRI in patients with metastatic colorectal cancer: KRAS mutation status correlates with clinical outcome. Ann Oncol 19(Suppl 6):vi25, Abstract PD-019. Updated information presented at the meeting
Raoul JL, Van Laethem JL, Peeters M, Brezault C, Husseini F, Cals L, Nippgen J, Loos AH, Rougier P (2009) Cetuximab in combination with irinotecan/5-fluorouracil/folinic acid (FOLFIRI) in the initial treatment of metastatic colorectal cancer: a multicentre two-part phase I/II study. BMC Cancer 9:112
Tabernero J, Van Cutsem E, Diaz-Rubio E, Cervantes A, Humblet Y, André T, Van Laethem J, Soulié P, Casado E, Verslype C, Valera JS, Tortora G, Ciardiello F, Kisker O, de Gramont A (2007) Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 25:5225–5232
Arnold D, Höhler T, Dittrich C, Lordick F, Seufferlein T, Riemann J, Wöll E, Herrmann T, Zubel A, Schmoll HJ (2008) Cetuximab in combination with weekly 5-fluorouracil/folinic acid and oxaliplatin (FUFOX) in untreated patients with advanced colorectal cancer: a phase Ib/II study of the AIO GI Group. Ann Oncol 19:1442–1449
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chung-Rong CC, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht F, Ruff R, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) FOLFIRI with and without cetuximab in the first-line treatment of metastatic colorectal cancer: efficacy, safety and KRAS analysis. N Engl J Med 360:1408–1417
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27:663–671
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634
Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC (2006) Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 55:657–670
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Van Cutsem E (2003) Cetuximab (C225) alone or in combination with irinotecan (CPT-11) in patients with Epidermal Growth Factor Receptor (EGFR)-positive, irinotecan-refractory metastatic colorectal cancer (MCRC). Proc Am Soc Clin Oncol 22:Abstract 1012. Virtual presentation: www.asco.org
Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201–1208
Van Cutsem E, Mayer RJ, Gold P, Stella PJ, Cohen A, Pippas AW, Windt P, Molloy P, Lenz H-J (2004) Correlation of acne rash and tumor response with cetuximab monotherapy in patients with colorectal cancer refractory to both irinotecan and oxaliplatin. EORTC-NCI-AACR Symposium Abstract 279. Updated information presented at meeting
Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB (2005) Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 23:1803–1810
Cappuzzo F, Finocchiaro G, Rossi E, Janne PA, Carnaghi C, Calandri C, Bencardino K, Ligorio C, Ciardiello F, Pressiani T, Destro A, Roncalli M, Crino L, Franklin WA, Santoro A, Varella-Garcia M (2008) EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients. Ann Oncol 19:717–723
Italiano A, Follana P, Caroli FX, Badetti JL, Benchimol D, Garnier G, Gugenheim J, Haudebourg J, Keslair F, Lesbats G, Lledo G, Roussel JF, Pedeutour F, Francois E (2008) Cetuximab shows activity in colorectal cancer patients with tumors for which FISH analysis does not detect an increase in EGFR gene copy number. Ann Surg Oncol 15:649–654
Personeni N, Fieuws S, Piessevaux H, De Hertogh G, De Schutter J, Biesmans B, De Roock W, Capoen A, Debiec-Rychter M, Van Laethem JL, Peeters M, Humblet Y, Van Cutsem E, Tejpar S (2008) Clinical usefulness of EGFR gene copy number as a predictive marker in colorectal cancer patients treated with cetuximab: a fluorescent in situ hybridization study. Clin Cancer Res 14:5869–5876
Malumbres M, Barbacid M (2003) RAS oncogenes: the first 30 years. Nat Rev Cancer 3:459–465
Andreyev HJ, Tilsed JV, Cunningham D, Sampson SA, Norman AR, Schneider HJ, Clarke PA (1997) K-ras mutations in patients with early colorectal cancers. Gut 41:323–329
Barry EL, Baron JA, Grau MV, Wallace K, Haile RW (2006) K-ras mutations in incident sporadic colorectal adenomas. Cancer 106:1036–1040
McLellan EA, Owen RA, Stepniewska KA, Sheffield JP, Lemoine NR (1993) High frequency of K-ras mutations in sporadic colorectal adenomas. Gut 34:392–396
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319:525–532
Benhattar J, Losi L, Chaubert P, Givel JC, Costa J (1993) Prognostic significance of K-ras mutations in colorectal carcinoma. Gastroenterology 104:1044–1048
Moerkerk P, Arends JW, van Driel M, de Bruine A, de Goeij A, ten Kate J (1994) Type and number of Ki-ras point mutations relate to stage of human colorectal cancer. Cancer Res 54:3376–3378
Belly RT, Rosenblatt JD, Steinmann M, Toner J, Sun J, Shehadi J, Peacock JL, Raubertas RF, Jani N, Ryan CK (2001) Detection of mutated K12-ras in histologically negative lymph nodes as an indicator of poor prognosis in stage II colorectal cancer. Clin Colorectal Cancer 1:110–116
Lee JC, Wang ST, Lai MD, Lin YJ, Yang HB (1996) K-ras gene mutation is a useful predictor of the survival of early stage colorectal cancers. Anticancer Res 16:3839–3844
Thebo JS, Senagore AJ, Reinhold DS, Stapleton SR (2000) Molecular staging of colorectal cancer: K-ras mutation analysis of lymph nodes upstages Dukes B patients. Dis Colon Rectum 43:155–159, discussion 159–162
Bouzourene H, Gervaz P, Cerottini JP, Benhattar J, Chaubert P, Saraga E, Pampallona S, Bosman FT, Givel JC (2000) p53 and Ki-ras as prognostic factors for Dukes’ stage B colorectal cancer. Eur J Cancer 36:1008–1015
Zauber NP, Wang C, Lee PS, Redondo TC, Bishop DT, Goel A (2004) Ki-ras gene mutations, LOH of the APC and DCC genes, and microsatellite instability in primary colorectal carcinoma are not associated with micrometastases in pericolonic lymph nodes or with patients’ survival. J Clin Pathol 57:938–942
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au H-J, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Richman SD, Chambers P, Elliott F, Daly C, Barrett J, Taylor G, Quirke P, Seymour M (2008) Prognostic and predictive value of KRAS and BRAF mutations in patients enrolled in the MRC FOCUS trial. Ann Oncol 19(Suppl 8):Abstract 5030. Updated information presented at the meeting
Etienne-Grimaldi MC, Formento JL, Francoual M, Francois E, Formento P, Renee N, Laurent-Puig P, Chazal M, Benchimol D, Delpero JR, Letoublon C, Pezet D, Seitz JF, Milano G (2008) K-Ras mutations and treatment outcome in colorectal cancer patients receiving exclusive fluoropyrimidine therapy. Clin Cancer Res 14:4830–4835
Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992–3995
Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG, Varmus HE (2005) KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2:e17
De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y, Van Cutsem E, Tejpar S (2008) KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 19:508–515
Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboue R, Tuech JJ, Queuniet AM, Paillot B, Sabourin JC, Michot F, Michel P, Frebourg T (2007) Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer 96:1166–1169
Finocchiaro G, Cappuzzo F (2007) EGFR, HER2, and Kras as predictive factors for cetuximab sensitivity in colorectal cancer. J Clin Oncol 25(18S):Abstract 4021
Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, Tan BR, Krishnamurthi SS, Burris HA 3rd, Poplin EA, Hidalgo M, Baselga J, Clark EA, Mauro DJ (2007) Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25:3230–3237
Lièvre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouche O, Landi B, Louvet C, Andre T, Bibeau F, Diebold MD, Rougier P, Ducreux M, Tomasic G, Emile JF, Penault-Llorca F, Laurent-Puig P (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26:374–379
Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, Siena S, Bardelli A (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67:2643–2648
Cappuzzo F, Varella-Garcia M, Finoccharia G, Skokan M, Gajapathy S, Carnaghi C, Rimassa L, Rossi E, Ligorio C, Di Tommaso L, Holmes AJ, Toschi L, Tallini G, Roncalli M, Santoro A, Janne PA (2008) Primary resistance to cetuximab therapy in EGFR FISH-positive colorectal cancer patients. Br J Cancer 99:83–89
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966
Ciuleanu TE, Scheithauer W, Kurteva G, Ocvirk J, Koza I, Papamichael D, Wenczl M, Brodowicz T, Beslija S, Zielinski CC (2008) A randomized, open-label phase II study evaluating the efficacy and safety of FOLFOX6 + cetuximab versus FOLFIRI + cetuximab as first-line therapy in patients (pts) with metastatic colorectal cancer (mCRC). ASCO Gastrointestinal Cancers Symposium: abstract 339
Rosenberg AH, Loehrer PJ, Needle MN, Waksal H, Hollywood E, Ramos L, Saltz LB (2002) Erbitux (IMC-C225) plus weekly irinotecan (CPT-11), fluorouracil (5FU) and leucovorin (LV) in colorectal cancer (CRC) that expresses the epidermal growth factor receptor (EGFr). Proc Am Soc Clin Oncol 21: abstract 536. Virtual presentation: www.asco.org
Borner M, Koeberle D, Von Moos R, Saletti P, Rauch D, Hess V, Trojan A, Helbling D, Pestalozzi B, Caspar C, Ruhstaller T, Roth A, Kappeler A, Dietrich D, Lanz D, Mingrone W (2008) Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann Oncol 19:1288–1292
Tabernero J, Cervantes A, Ciardiello F, Vega-Villegas E, Macarulla T, Rodriguez-Braun E, Martinelli E, Stroh C, Nippgen J, Baselga J (2008) Correlation of efficacy to KRAS status (wt vs. mut) in patients (pts) with metastatic colorectal cancer (mCRC), treated with weekly (q1w) and q2w schedules of cetuximab combined with FOLFIRI. ASCO Gastrointestinal Cancers Symposium January 25–27, Orlando, FL, USA, abstract 435. Updated information presented at meeting
Koza I, Wrba F, Vrabanec D, Ocvirk J, Ciuleanu TE, Beslija S, Papamichael D, Messinger C, Zielinski CC, Brodowicz T (2009) Correlation of KRAS status with clinical outcome in patients (pts) with metastatic colorectal cancer (mCRC) treated first-line with FOLFOX6 + cetuximab (FX+C) or FOLFIRI + cetuximab (FF+C): The CECOG/CORE 1.2.001 trial. J Clin Oncol 27(15S): abstract 2055. Updated information presented at meeting
Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, Ince WL, Janne PA, Januario T, Johnson DH, Klein P, Miller VA, Ostland MA, Ramies DA, Sebisanovic D, Stinson JA, Zhang YR, Seshagiri S, Hillan KJ (2005) Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 23:5900–5909
Adam R, Wicherts DA, de Haas RJ, Ciacio O, Levi F, Paule B, Ducreux M, Azoulay D, Bismuth H, Castaing D (2009) Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 27:1829–1835
Folprecht G, Grothey A, Alberts S, Raab HR, Köhne CH (2005) Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 16:1311–1319
Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, Schwartzberg L, Hedrick E (2008) Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 26:3523–3529
Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O (2009) The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist 14:22–28
Cassidy J, Cunningham D, Berry SR, Rivera F, Clarke SJ, Kretzschmar A, Diaz-Rubio E, Van Cutsem E, Saltz LB (2008) Surgery with curative intent in patients (pts) treated with first-line chemotherapy (CT) + bevacizumab (BEV) for metastatic colorectal cancer (mCRC): First BEAT and NO16966. J Clin Oncol 26(May 20 suppl): abstract 4022
Bechstein W, Lang H, Kabbinavar F, Köhne CH, Parisi F, Raab H-R, Frilling A, Konopke R, Weitz J, Stroszczynski C, Folprecht F (2009) Resectability and agreement between surgeons: Review of CT and MR scan of the CELIM study: (Multicenter randomized trial of cetuximab/FOLFOX versus cetuximab/FOLFIRI in unresectable liver metastases). J Clin Oncol 27(15S): abstract 4055. Updated information presented at meeting
NICE. Final appraisal determination. Cetuximab for the first-line treatment of metastatic colorectal cancer. http://www.nelm.nhs.uk/en/NeLM-Area/News/2009—June/02/NICE-issues-FAD-for-cetuximab-for-the-first-line-treatment-of-metastatic-colorectal-cancer/Post.aspx. Accessed 24 June 2009
Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, Papadimitriou CA, Murray S (2008) Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol 9:962–972
Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A (2008) Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 26:5705–5712
Lambrechts D, De Roock W, Prenen H, De Schutter B, Jacobs B, Biesmans B, Claes G, De Hertogh E, Van Laethem JL, Tejpar S (2009) The role of KRAS, BRAF, NRAS, and PIK3CA mutations as markers of resistance to cetuximab in chemorefractory metastatic colorectal cancer. J Clin Oncol 27(15S): abstract 4020
Lea IA, Jackson MA, Li M, Bailey S, Peddada SD, Dunnick JK (2007) Genetic pathways and mutation profiles of human cancers: site- and exposure-specific patterns. Carcinogenesis 28:1851–1858
Ruzzo A, Cremolini C, Loupakis F, Fornaro L, Santini D, Vincenzi B, Canestrari E, Magnani M, Falcone A, Graziano F (2009) Association of BRAF mutations and EGFR Intron-1 L/L genotype with resistance to cetuximab plus irinotecan treatment in KRAS wild-type metastatic colorectal cancer patients. J Clin Oncol 27(15S): abstract 4058
Kohne C, Stroiakovski D, Chang-Chien C, Lim R, Pintér T, Bodoky G, Stroh C, Celik I, Rougier P, Van Cutsem E (2009) Predictive biomarkers to improve treatment of metastatic colorectal cancer (mCRC): outcomes with cetuximab plus FOLFIRI in the CRYSTAL trial. J Clin Oncol 27(15S): abstract 4068. Virtual presentation at www.asco.org
Jonker DJ, Karapetis CS, Harbison C, O’Callaghan CJ, Tu D, Simes RJ, Xu L, Moore MJ, Zalcberg JR, Khambata-Ford S (2009) High epiregulin (EREG) gene expression plus K-ras wild-type (WT) status as predictors of cetuximab benefit in the treatment of advanced colorectal cancer (ACRC): Results from the NCIC CTG CO.17-A phase III trial of cetuximab versus best supportive care (BSC). J Clin Oncol 27(15S): abstract 4016
Loupakis F, Cremolini C, Perrone G, Stasi I, Salvatore L, Vincenzi B, Ruzzo A, Ronzoni M, Bencardino K, Falcone A (2009) Amphiregulin (AR) expression in the prediction of benefit from cetuximab plus irinotecan in KRAS wild type metastatic colorectal cancer (mCRC) patients. J Clin Oncol 27(15S): abstract 4021
Prenen H, Jacobs B, De Roock W, Van Oirbeek R, Biesmans B, De Schutter J, Fieuws S, Laurent-Puig P, Van Cutsem E (2009) Use of amphiregulin and epiregulin mRNA expression in primary tumors to predict outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 27(15S): abstract 4019
Oden-Gangloff A, Di Fiore F, Bibeau F, Lamy A, Bougeard G, Charbonnier F, Blanchard F, Tougeron D, Ychou M, Boissiere F, Le Pessot F, Sabourin JC, Tuech JJ, Michel P, Frebourg T (2009) TP53 mutations predict disease control in metastatic colorectal cancer treated with cetuximab-based chemotherapy. Br J Cancer 100:1330–1335
Frattini M, Saletti P, Romagnani E, Martin V, Molinari F, Ghisletta M, Camponovo A, Etienne LL, Cavalli F, Mazzucchelli L (2007) PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer 97:1139–1145
Loupakis F, Pollina L, Stasi I, Ruzzo A, Scartozzi M, Santini D, Masi G, Graziano F, Cremolini C, Rulli E, Canestrari E, Funel N, Schiavon G, Petrini I, Magnani M, Tonini G, Campani D, Floriani I, Cascinu S, Falcone A (2009) PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol 27:2622–2629
Perrone F, Lampis A, Orsenigo M, Di Bartolomeo M, Gevorgyan A, Losa M, Frattini M, Riva C, Andreola S, Bajetta E, Bertario L, Leo E, Pierotti MA, Pilotti S (2008) PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol 20:84–90
Razis E, Briasoulis E, Vrettou E, Skarlos DV, Papamichael D, Kostopoulos I, Samantas E, Xanthakis I, Bobos M, Galanidi E, Bai M, Gikonti I, Koukouma A, Kafiri G, Papakostas P, Kalogeras KT, Kosmidis P, Fountzilas G (2008) Potential value of PTEN in predicting cetuximab response in colorectal cancer: an exploratory study. BMC Cancer 8:234
National Comprehensive Cancer Network updates guidelines for colorectal cancer. www.nccn.org/about/news/newsinfo.asp?NewsID=194. Accessed April 2009
Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL (2009) American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 27:2091–2096
van Krieken JH, Jung A, Kirchner T, Carneiro F, Seruca R, Bosman FT, Quirke P, Flejou JF, Hansen TP, de Hertogh G, Jares P, Langner C, Hoefler G, Ligtenberg M, Tiniakos D, Tejpar S, Bevilacqua G, Ensari A (2009) KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: proposal for an European quality assurance program. Virchows Arch 454:233–235
van Krieken JH, Jung A, Kirchner T, Carneiro F, Seruca R, Bosman FT, Quirke P, Flejou JF, Plato Hansen T, de Hertogh G, Jares P, Langner C, Hoefler G, Ligtenberg M, Tiniakos D, Tejpar S, Bevilacqua G, Ensari A (2008) KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: proposal for an European quality assurance program. Virchows Arch 453:417–431
Acknowledgements
The authors wish to acknowledge the input of the members of the panel. These were: Ass. Prof. Assen Dudov, Sofia Cancer Center, Sofia, Bulgaria; Ass. Prof. Stjepko Plestina, Clinical Hospital Center, Zagreb, Croatia; Ass. Prof. Evangelos Briassoulis, University Hospital, Ioannina, Greece; Ass. Prof. Demetrios Mavroudis, University Hospital of Heraklion, Crete, Greece; Prof. George Fountzilas, University Hospital of Thessaloniki “Papageorgiou”, Thessaloniki, Greece; Samuel Murray PhD, Head of Molecular Oncology and Genetics, Metropolitan Hospital, Athens, Greece; Prof Baruch Klein, Meir Medical Center, Kfar Saba, Israel; Ass. Prof Tudor Ciuleanu, Institutut Oncologic ‘Ion Chricuta’ Cluj, Cluj Napoca 400015, Romania; Dr Dusan Jovanovic, Institute of Oncology Sremska Kamenica, Novi Sad, Serbia; Dr Jana Ocvirk, Institute of Oncology, Ljubljana, Slovenia: Prof. Gokhan Demir, Florence Nigthingale Hospital, Istanbul, Turkey.
Conflict of interest statement
Although the authors have not received and will not receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this manuscript, benefits have been or will be received, but are directed solely to a research fund, foundation, educational institution or other non-profit organization with which one or more of the author(s) is associated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciardiello, F., Tejpar, S. & Papamichael, D. Implications of KRAS mutation status for the treatment of metastatic colorectal cancer. Targ Oncol 4, 311–322 (2009). https://doi.org/10.1007/s11523-009-0129-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-009-0129-6