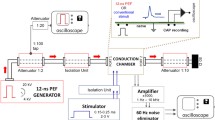
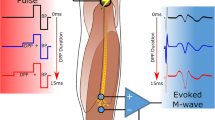
Abstract
The number of devices for electrical stimulation of nerve fibres implanted worldwide for medical applications is constantly increasing. Stimulation charge is one of the most important parameters of stimulation. High stimulation charge may cause tissue and electrode damage and also compromise the battery life of the electrical stimulators. Therefore, the objective of minimizing stimulation charge is an important issue. Delaying the second phase of biphasic stimulation waveform may decrease the charge required for fibre activation, but its impact on stimulation selectivity is not known. This information is particularly relevant when transverse intrafascicular multichannel electrode (TIME) is used, since it has been designed to provide for high selectivity. In this in vivo study, the rat sciatic nerve was electrically stimulated using monopolar and bipolar configurations with TIME. The results demonstrated that the inclusion of a 100-μs delay between the cathodic and the anodic phase of the stimulus allows to reduce charge requirements by around 30 %, while only slightly affecting stimulation selectivity. This study shows that adding a delay to the typical stimulation waveform significantly (\(P < 0.001\)) reduces the charge required for nerve fibres activation. Therefore, waveforms with the delayed discharge phase are more suitable for electrical stimulation of nerve fibres.





Similar content being viewed by others
References
Abdi H (2007) The Bonferonni and Šídák corrections for multiple comparisons. In: Salkind NJ (ed) Encyclopedia of measurement and statistics. Sage, Thousand Oaks, pp 103–107
Arle JE (2011) The neuromodulation approach. In: Arle JE, Shils JL (eds) Essential neuromodulation. Academic Press, Waltham, pp 1–16
Andreu D, Guiraud D, Souquet G (2009) A distributed architecture for activating the peripheral nervous system. J Neural Eng 6:026001
Badia J, Pascual-Font A, Vivó M, Udina E, Navarro X (2010) Topographical distribution of motor fascicles in the sciatic–tibial nerve of the rat. Muscle Nerve 42:192–201
Badia J, Boretius T, Andreu D, Azevedo-Coste C, Stieglitz T, Navarro X (2011) Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicle. J Neural Eng 8:036023
Badia J, Boretius T, Pascual-Font A, Udina E, Stieglitz T, Navarro X (2011) Biocompatibility of chronically implanted transverse intrafascicular multichannel electrode (TIME) in the rat sciatic nerve. IEEE Trans Biomed Eng 58:2324–2332
Del Valle J, Navarro X (2013) Interfaces with the peripheral nerve for the control of neuroprostheses. Int Rev Neurobiol 109:63–83
Gorman PH, Mortimer JT (1983) The effect of stimulus parameters on the recruitment characteristics of direct nerve stimulation. IEEE Trans Biomed Eng 30:407–14
Grill WM, Mortimer JT (1995) Stimulus waveforms for selective neural stimulation. IEEE Trans Biomed Eng 14:375–85
Guiraud D, Stieglitz T, Taroni G, Divoux JL (2006) Original electronic design to perform epimysial and neural stimulation in paraplegia. J Neural Eng 3:276–86
Hofmann L, Ebert M, Tass PA, Hauptmann C (2011) Modified pulse shapes for effective neural stimulation. Front Neuroeng 4:9
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:6570
Kundu A, Harreby K, Yoshida K, Boretius T, Stieglitz T, Jensen W (2014) Stimulation selectivity of the thin-film longitudinal intrafascicular electrode (tfLIFE) and the transverse intrafascicular multi-channel electrode (TIME) in the large nerve animal model. IEEE Trans Neural Syst Rehabil Eng 22:400–410
Maciejasz P, Badia J, Boretius T, Harreby K, Jensen W, Stieglitz T, Navarro X, Guiraud D (2013) Comparison of stimulation selectivity in monopolar and bipolar configuration using the transversal intrafascicular multichannel electrode (TIME)—preliminary results. In: Pons JL, Torricelli D, Pajaro M (eds) Converging clinical and engineering research on neurorehabilitation, vol 1., Biosystems and BioroboticsSpringer, Berlin, pp 79–83
Marin J, De Lannoy G, Delbeke J (2009) When can we recover charges with a biphasic charge balanced stimulation pulse? In: Proceedings of the international functional electrical stimulation society conference, Seoul, Korea, pp 55–57
Merrill DR, Bikson M, Jefferys JGR (2005) Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods 141:171–98
Prado-Guitierrez P, Fewster LM, Heasman JM, McKay CM, Shepherd RK (2006) Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res 215:4755
Prodanov D, Marani E, Holsheimer J (2003) Functional electric stimulation for sensory and motor functions: progress and problems. Biomed Rev 14:23–50
Raspopovic S, Capogrosso M, Badia J, Navarro X, Micera S (2012) Experimental validation of a hybrid computational model for selective stimulation using transverse intrafascicular multichannel electrodes. IEEE Trans Neural Syst Rehabil Eng 20:395–404
Rattay F (1986) Analysis of models for external stimulation of axons. IEEE Trans Biomed Eng 33:974–77
Schultz AE, Kuiken TA (2011) Neural interfaces for control of upper limb prostheses: the state of the art and future possibilities. PMR 3:55–67
Shepherd RK, Javel E (1999) Electrical stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear Res 130:17188
Stieglitz T, Boretius T, Navarro X, Badia J, Guiraud D, Divoux JL, Micera S, Rossini PM, Yoshida K, Harreby KR, Kundu A, Jensen W (2012) Development of a neurotechnological system for relieving phantom limb pain using transverse intrafascicular electrodes (TIME). Biomed Tech (Berl) 57:457–465
van den Honert C, Mortimer JT (1979) The response of the myelinated nerve fiber to short duration biphasic stimulating currents. Ann Biomed Eng 7:117–25
Veraart C, Grill WM, Mortimer JT (1993) Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans Biomed Eng 40:640–53
Weitz AC, Behrend MR, Ahuja AK, Christopher P, Wei J, Wuyyuru V, Patel U, Greenberg RJ, Humayun MS, Chow RH, Weiland JD (2014) Interphase gap as a means to reduce electrical stimulation thresholds for epiretinal prostheses. J Neural Eng 11:016007
Wright SP (1992) Adjusted P-values for simultaneous inference. Biometrics 48:1005–1013
Acknowledgments
We would like to thank Mr. Guillaume Souquet from MXM Axonic for developing low-level control software of the Stim’nD stimulator, Mr. François Bonnetblanc from the DEMAR team, INRIA, for help with statistical analysis and Ms. Chloé Picq from MXM Axonic for English proof reading.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by the European Union through the FP7 Projects: EPIONE—“Natural sensory feedback for phantom limb pain modulation and therapy” (Project Number: 602547), and TIME—“Transverse, Intrafascicular Multichannel Electrode system for induction of sensation and treatment of phantom limb pain in amputees” (Project Number: 224012).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maciejasz, P., Badia, J., Boretius, T. et al. Delaying discharge after the stimulus significantly decreases muscle activation thresholds with small impact on the selectivity: an in vivo study using TIME. Med Biol Eng Comput 53, 371–379 (2015). https://doi.org/10.1007/s11517-015-1244-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-015-1244-4